Premia Spine Launches TOPS™ System IDE study
First posterior arthroplasty device for degenerative grade I spondylolisthesis and spinal stenosis
PHILADELPHIA, PENNSYLVANIA, USA, July 19, 2017 /EINPresswire.com/ -- Premia Spine, Ltd. announced today that it has launched its FDA pivotal study of the new TOPS™ System. The first surgery was performed by Dr. Steven DeLuca of the Orthopedic Institute of Pennsylvania. The TOPS™ procedure was performed at Pinnacle Westshore Hospital in Harrisburg, Pennsylvania."I am very pleased with the ease of the procedure and the immediate post-operative result,” stated Dr. DeLuca.
“We are excited about the opportunity to provide U.S. patients with access to the only posterior arthroplasty device for degenerative grade I spondylolisthesis and spinal stenosis, with thickening of the ligament or scarring of the facet capsule," said Ron Sacher, CEO of Premia Spine.
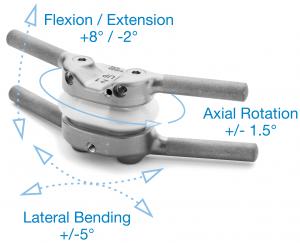
The new TOPS device, with a 30% smaller footprint and a simpler surgical technique from the original device, has been in commercial use in Europe for over 5 years.
The IDE study will take place in 30 institutions and enroll 330 subjects. Patients will be randomized to either the TOPS™ System or lumbar fusion (i.e., an interbody cage plus screws and rods), with a 67% likelihood of receiving the TOPS device.
Clinical sites will be measuring ODI, VAS, neurologic function, device integrity, reoperation rates and other quantitative outcomes for the study device and the fusion control. "Our goal is to establish the superiority of the TOPS™ System versus traditional lumbar spinal fusion," explains Mr. Sacher.
About Premia Spine. Premia Spine licensed the TOPS System technology in 2011 from Impliant, Ltd. Over $100 million has been invested to design, develop, and commercialize the TOPS System, with over 12 years of clinical use and 1,000 patients.
Ron Sacher
Premia Spine
/
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.