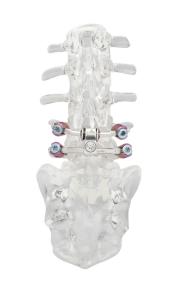
Premia Spine releases Nexux™ System in Germany for Spinal Instability
Novel posterior motion device maintains lumbar stability even after decompression
MUNICH, GERMANY, July 13, 2017 /EINPresswire.com/ -- Premia Spine GmbH announced today the full market release of the Nexux™ System for the treatment of lumbar spinal instability."We are excited about the opportunity to be the first to provide patients in Germany with access to the only posterior arthroplasty device that solves the problem of lumbar instability," said Kiarash Pourbagher, Vice President, Sales and Marketing Germany.
The Nexux device is part of the TOPS™ System family of products from Premia Spine. The new implant is smaller and modular, allowing surgeons to perform a less invasive procedure.
"I find the instrumentation very intuitive and easy to use," Dr. Thomas Bierstedt, Neurosurgeon and Senior Consultant at the ONZ Datteln/Recklinghausen, highlighted after his first case with the new system. His partner and Senior Consultant at ONZ, Dr. Bernd Illerhaus added that "the Nexux System is more bone sparing, allowing surgeons to maintain the lamina and undercut the posterior elements to free impinged nerve roots."
The Nexux System was introduced in BKH Günzburg as well, where Dr. Christoph Grimm performed his first case with the new instrumentation. "I see many patients who suffer from instability and require instrumentation to control their slip. Nexux is a good alternative for surgeons who want to maintain motion at the operative segment instead of fusion," remarked Dr. Grimm.
Other centers across Germany which have been waiting for the Nexux System will be trained and provided access to the new system in the coming weeks.
"Premia Spine continues to look for opportunities to bring clinically meaningful solutions to lower back pain suffers," explains Mr. Sacher. "The Nexux is another innovative way for patients to avoid spinal fusion when a motion alternative is available."
About Premia Spine. Premia Spine licensed the TOPS System technology in 2011 from Impliant, Ltd. Over $100 million has been invested to design, develop, and commercialize the Company's product line, with over 12 years of clinical use and 1,000 patients.
Ron Sacher
Premia Spine
/
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.