Understanding How Neurofilaments Clog Up Brain Functions
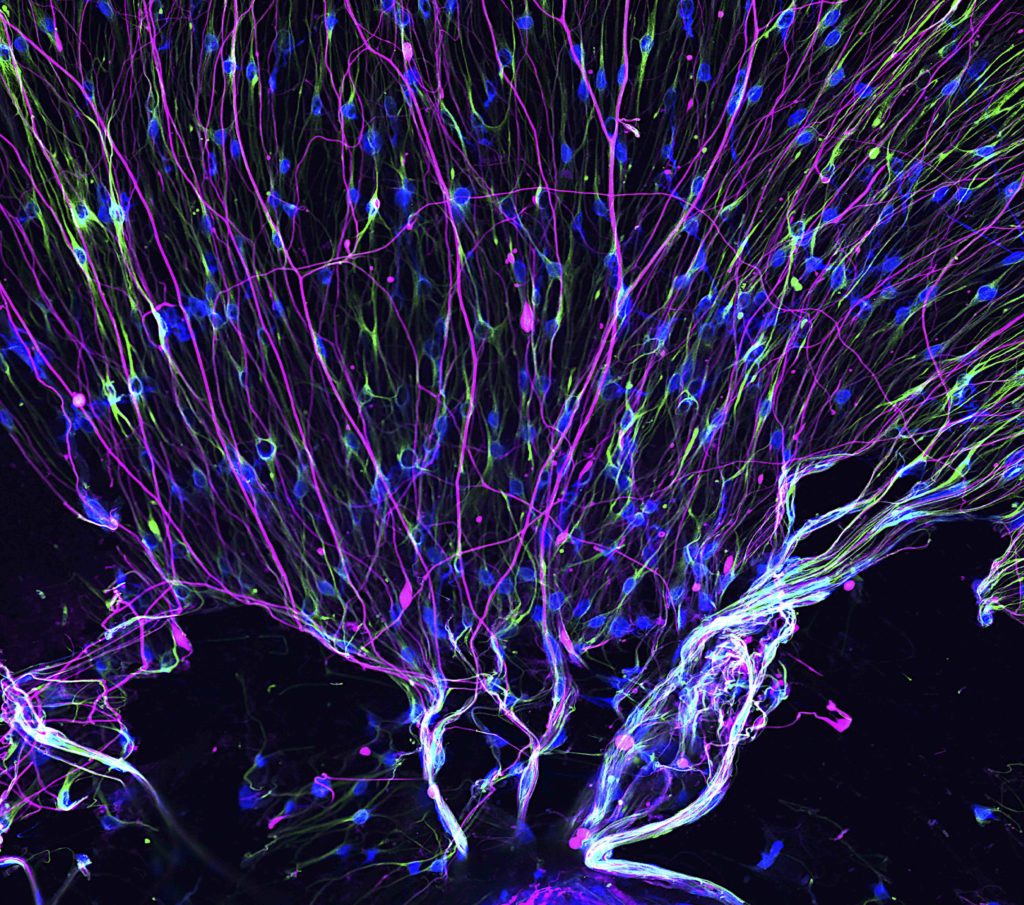
Northwestern Medicine scientists have uncovered new insights into how neurofilaments act like Velcro in neurodegenerative diseases, clogging up the brain and preventing normal function, according to a study published in the journal JCI Insight.
Giant axonal neuropathy (GAN) is a rare, genetic neurodegenerative disorder in which large nerve fibers in the brain (axons) abnormally accumulate clumps of cytoskeletal proteins called neurofilaments. Neurofilaments have also been linked to multiple neurodegenerative disorders, including Parkinson’s Disease, dementia and amyotrophic lateral sclerosis (ALS).

Previous research has shown that the buildup of neurofilaments in GAN is caused by a lack of gigaxonin, a protein that helps break down filaments. However, the exact mechanisms that control this process have not been well understood, said Puneet Opal, MD, ‘95 PhD, the Lewis John Pollock Professor of Neurology in the Division of Movement Disorders and of Cell and Developmental Biology, and senior author of the study.
“We wanted to understand whether there could be an alternative pathway for degrading proteins which could be upregulated when the gigaxonin pathway is mutated,” said Opal, who also directs the Denning Ataxia Center.
In the study, Opal and his collaborators employed a combination of genetic and RNA interference (RNAi) approaches to study the brains of mice which lacked gigaxonin.
They observed that the cellular recycling processes were disrupted because the neurofilaments acted like Velcro in the brain, preventing organelles from moving, according to the study.
Additionally, the cellular recycling plants – called lysosomes – were missing key digestive enzymes responsible for breaking down waste products, the scientists found.
“These vesicles can’t reach the garbage can – lysosomes,” Opal said. “So, the neurofilaments cannot be degraded by that. Additionally, the neurofilaments create docking sites for proteins and organelles normally, but in the disease, these sites are a little bit garbled.”
By monitoring lysosomal activity in mice with GAN, the investigators also found that Transcription factor EB (TFEB), a protein essential for the creation of healthy lysosomes, became trapped in the neurofilaments, preventing it from moving around the cell.
“This dual problem basically causes a snowballing effect where the protein cannot be degraded by its normal pathway,” Opal said. “This explains why children with this disease become worse and worse with age.”
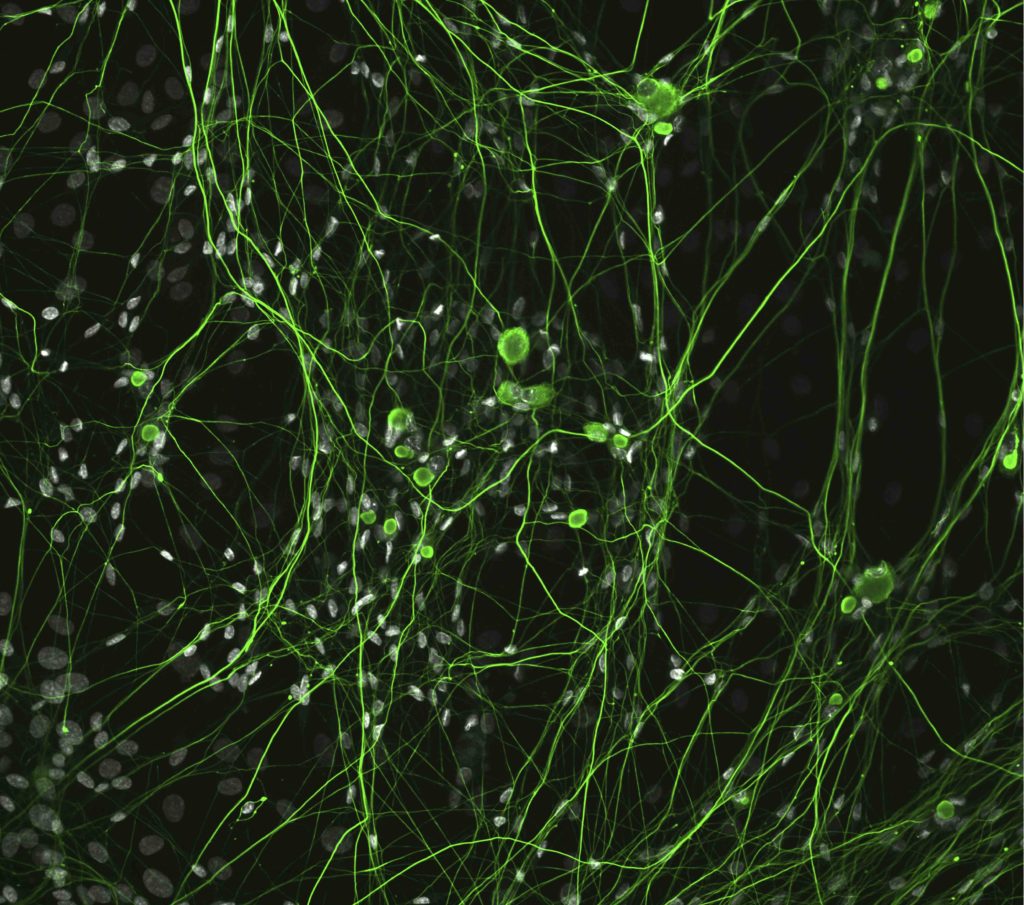
The findings are also useful for understanding other neurodegenerative diseases which feature neurofilament accumulations, such as Parkinson’s and Alzheimer’s disease, Opal said.
“When you have these other diseases, when so much else is going on, it’s difficult to pinpoint the role of neurofilaments,” Opal said. “But here, with this genetic disease, we basically honed in on this very singular pathway.”
On the heels of this discovery, Opal said he and his collaborators hope to create new drugs which can reverse the accumulation of neurofilaments.
Jeffrey Savas, PhD, associate professor in the Ken and Ruth Davee Department Neurology in the Division of Behavioral Neurology, was a co-author of the study.
The study was supported by the Giddan Foundation, the Muscular Disease Association, National Institute of Neurological Disorders and Stroke grants (1R01NS127204-01 and R01NS082351) and prior seed funding from Hannah’s Hope Fund.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
