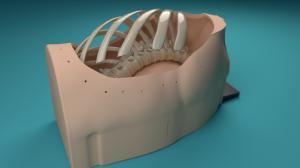
21st Century Surgical Training Education is Driving Medical Device Success & Regulatory Approvals
Surgical Training and Education: Encoris' State-of-the-Art Surgical Simulation Products Provide Realistically Immersive Experiences for Effective Learning.
HOLLAND, MI, UNITED STATES, December 21, 2023 /EINPresswire.com/ -- Encoris Group Corporation plays a leading role in shaping the future of the medical device industry, providing innovative, effective, and efficient tools for surgical training globally.As medical science continues developing cutting-edge implant technologies, Encoris Group Corporation stands out as a leader of innovation in surgical training education—an indispensable part of launching new medical devices. Encoris' high-tech development and manufacturing capabilities create highly interactive and engaging training modules that enhance and accelerate the surgical learning experience and industry adoption of new medical devices.
Headquartered in Holland, MI, Encoris Group Corporation is a leading designer and manufacturer of 21st-century medical models and surgical trainers worldwide. Incorporating cutting-edge technology, vast material options, and novel innovation into the development of its simulated surgical training products, the company's approach and commitment to excellence in surgical training education is renowned globally, preparing countless surgeons, fellows, medical device companies and their medical device reps for the challenges of today's healthcare environment.
For more information, visit https://encoris.com/surgical-training-education/
Encoris recognizes the critical role surgical training education plays in the total success of implant adoption. Medical device companies that provide credible surgical training models and education protocols foster a greater understanding and commitment to advancing surgical care and medical science.
When launching a new device, anatomical accuracy and speed to market are of utmost importance. Encoris' expertise in CAD and product development accelerates the manufacturing process, producing quality products with the fastest lead times in the industry, helping clients introduce and launch new medical device technologies more quickly to satisfy ever-increasing surgical training demands and sales and marketing objectives.
Encoris' 20+ years of surgical training education experience and industry knowledge are highly respected and appreciated among medical institutions and corporate heavyweights like Stryker, DePuy Synthes, Zimmer Biomet, Medtronic, and many up-and-coming leading medical device companies. Keen market insight drives Encoris' innovative spirit, which enriches the educational efforts of its medical device clientele to ensure they remain at the forefront of surgical advancements.
Encoris' approach to surgical training education efficacy extends far beyond the individual trainee but impacts the entire industry. A well-trained surgeon performs safer surgeries, significantly reducing surgical complications and time in the OR and improving patient healing. In this way, Encoris contributes significantly to elevating the standards of healthcare services and patient care overall.
Youtube embed: https://www.youtube.com/watch?v=1xZ5NZfibE8.
With an increasing demand for products that enhance technical skills development, proficient surgical training education instills greater competence and confidence, promoting higher-quality surgical procedural outcomes.
Encoris' customized surgical training education products are not a one-size-fits-all product. Training Phantoms can be customized to include pathology and features that further enhance and highlight a medical device's key features and benefits. Encoris' sophisticated products provide a better understanding and acceptance of medical devices, invigorating surgical skills training and spotlighting sales-rep-to-surgeon and surgeon-patient interactions.
Cadavers are limited in supply, are costly to use, biohazardous, and do not produce favorable anatomic results - a system of training that continues to be a burden to minimally invasive surgical (MIS) innovation. Encoris' cutting-edge products use the latest technologies to propel and satisfy the demands of today's surgical training education needs. 3D printing, embedded sensory feedback, customized pathology-specific anatomy, radiation-free X-ray imagery, augmented Reality (AR), and remote work communications advancements provide greater freedom and allow trainees to better practice and refine intricate surgical techniques outside traditional cadaver labs.
Encoris Group Corporation's focus on innovative, customized solutions sets it apart from the industry. By understanding a medical device company's unique educational goals and challenges, Encoris can meticulously design and craft training models that meet specific implant requirements, no matter how elusive. This personalized approach ensures that a medical device company's medical education objectives are targeted, focused, maximized, and refined when introducing new medical devices. Encoris understands that first impressions mean everything.
Encoris' services also lend a hand to implant product development and regulatory compliance. The integration of medical models significantly elevates the assessment of engineering usability, aligning seamlessly with the principles of BS EN 62366:2015 +A1 2020. This standard delineates a comprehensive process for manufacturers to scrutinize, specify, develop, and evaluate the usability of medical devices, prioritizing safety. Usability engineering, also referred to as human factors engineering (HFE), plays a pivotal role in designing user interfaces that foster efficient and error-free interactions. Our models seamlessly integrate into the usability engineering process, offering manufacturers a dynamic platform to simulate and assess user-device interactions. This enables a meticulous analysis of correct use and potential errors during normal scenarios, facilitating the identification and mitigation of usability-associated risks. The iterative nature of medical modeling allows for continuous refinement, ensuring the final product aligns with contemporary usability engineering concepts, per the updated BS EN 62366:2015 +A1 2020 standard. In conjunction with its companion document, IEC 62366-2, this standard replaces the previous edition and provides manufacturers with a robust framework for addressing usability considerations. It strengthens the link to risk management methodologies specified in ISO 14971:2019+ A11 2021. In essence, utilizing medical models within the context of BS EN 62366 fosters a more thorough and effective evaluation of engineering usability, ultimately contributing to developing safer and more user-friendly medical devices.
About the Company:
Encoris Group Corporation’s pursuit of excellence in surgical training education has made it an indispensable part of the medical device industry. Through innovation, state-of-the-art technologies, and the ability to collaborate easily with customers worldwide, Encoris continues to shape the future of surgical education, helping medical device companies and surgeons drive the industry toward a safer, more efficient, and patient-centered future. Encoris' impact on surgical training education resonates far and wide, making it a cornerstone in the ever-evolving field of medical implants.
==================================
Contact Details:
Company Name: Encoris Group Corporation
Client's Name: Jim TenBrink, Co-Founder, VP
Address: 180 Roost Ct. Holland, MI 49424
Email: jtenbrink@encoris.com
Phone: 1-800-957-7137
Website: https://encoris.com/surgical-training-education/
Regulatory: MDR Consultants Inc., Miami, Florida P: 786.306.0227 email: info@mdrconsultants.com
Jim TenBrink
Encoris Group Corporation
+1 616-335-2000
email us here
Visit us on social media:
YouTube
Other
Surgical Training Education: Why Working with Encoris Catapults the Success of Your Medical Devices
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.