SimcereZaiming showcases latest R&D progressed on its debut at ASCO, as 6 studies regarding 3 products published at ASCO
Simcere Zaiming, an innovative oncology-focused biopharma and a subsidiary of Simcere Pharmaceutical Group, exhibited a booth at ASCO for the first time.
BOSTON, MASSACHUSETTS, THE UNITED STATES, June 6, 2023/EINPresswire.com/ -- The annual meeting of the American Society of Clinical Oncology (ASCO) 2023, one of the most prestigious clinical academic events in oncology, is being held in Chicago, USA on June 2-6. Simcere Zaiming, an innovative oncology-focused biopharmaceutical company and a subsidiary of Simcere Pharmaceutical Group, exhibited a booth at ASCO for the first time. The Zaiming team held on-site meetings with clinicians from around the world to discuss the progress being made by the company by showcasing the latest he research and development of the company’s innovative anti-tumor drugs.A total of six relevant studies regarding three innovative drugs in Zaiming’s product portfolio, COSELA ®, Enweida ® and Endostar ® , have published the latest clinical progress in the form of posters and abstracts. Among them, there are clinical trials initiated by Chinese and foreign investigators (IIT), real-world studies (RWS), and registration studies promoted by Zaiming collaborative innovation partners, involving small cell lung cancer, non-small cell lung cancer, triple-negative breast cancer, gastric cancer, soft tissue sarcoma, and other cancer types. Congratulations to the Zaiming team!
COSELA® (trilaciclib) is an innovative bone marrow protection drug to be used prior to chemotherapy, developed by Simcere Pharmaceutical Co., Ltd. in cooperation with G1 Therapeutics of the United States. COSELA has been approved for marketing in China and the United States. Simcere Zaiming has the rights and interests of all indications of this product in Greater China
01 Real world outcomes of trilaciclib in ES-SCLC
Abstract No.: e20637
Author: Joseph Elijah et al., Roswell Park Comprehensive Cancer Center
https://meetings.asco.org/abstracts-presentations/223649
02 Neoadjuvant single-dose trilaciclib prior to combination chemotherapy in patients with early triple-negative breast cancer: Safety, efficacy, and immune data from a phase 2 study
Abstract No.: 603
Poster No.: 433
Author: Jeremy Meyer Force et al., Duke University School of Medicine
https://meetings.asco.org/abstracts-presentations/223763
Enweida ® (Envafolimab) is an innovative subcutaneous injection PD-L1 antibody tumor drug from Simcere Pharmaceutical Group in strategic cooperation with 3Dmed and Alphamab, and Simcere Zaiming is responsible for the exclusive commercial promotion of the product in mainland China
01 First-line envafolimab plus SOX chemotherapy for PD-L1 positive metastatic or recurrent gastric adenocarcinoma: A multi-centre, single-arm phase II clinical trial.
Abstract No.: e16029
Author: Zhu Liangjun, Jiangsu Cancer Hospital
https://meetings.asco.org/abstracts-presentations/222055
02 ENVASARC: A pivotal trial of envafolimab and envafolimab in combination with ipilimumab in patients with advanced or metastatic unpleomorphic sarcoma or myxofibrosarcoma who have progressed on prior chemotherapy.
Abstract No.: TPS11583
Poster No.: 515a
Author: Richard F. Riedel et al. Duke Cancer Institute, Duke University Medical Center
https://meetings.asco.org/abstracts-presentations/226102
Endostar ® (recombinant human endostatin) is a Simcere and then Lumenis product, a angiogenesis inhibitor
01 Endostatin in combination with PD-1 antibody plus chemotherapy as first-line regimen for EGFR/ALK-negative, advanced or metastatic non-small cell lung cancer: A real-world study
Abstract No.: e20564
Author: Zhang Guoqing, et al., First Medical Center, Chinese PLA General Hospital
https://meetings.asco.org/abstracts-presentations/223582
02 Immunotherapy combined with rh-endostatin improved clinical outcomes over ICIs plus chemotherapy for second-line treatment of advanced NSCLC.
Abstract No.: e21133
Author: Hongxiang Huang et al., Department of Oncology, the First Affiliated Hospital of Nanchang University
https://meetings.asco.org/abstracts-presentations/219073
About Simcere Zaiming
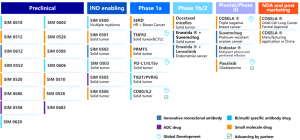
Simcere Zaiming is a pharmaceutical company specializing in oncology, and a subsidiary of Simcere Pharmaceutical Group Limited, that focuses on the R&D, production and commercialization of innovative cancer therapeutics. The company was formed in 2023 and is committed to solving unmet clinical needs for cancer patients in China and around the world by developing breakthrough treatments. Simcere Zaiming has an innovative R&D pipeline with differentiated clinical value, among which 15 assets are currently in clinical trials. In addition to the company’s R&D portfolio, Simcere Zaiming is marketing three innovative drugs, COSELA®, Endostar®, and Envafolimab®. By collaborating with partners globally, Simcere Zaiming strives to bring potentially new innovative therapeutics to cancer patients worldwide.
Media Contact: pr@zaiming.com
Yiling Li
Simcere Zaiming Pharmaceutical Co.,Ltd.
+86 139 1475 7210
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.