Palisades Therapeutics Announces New Anti-Cancer Drug-PT162, a p53-Reactivating Cell Cycle Checkpoint Inhibitor
Newly Patented PT162 Induces Cancer Cell Cycle Arrest and/or Apoptosis by Restoring DNA-Binding Activity of Mutant p53 Protein
Palisades Therapeutics’ PT162 accomplishes that goal and, like recent discoveries of inflammatory checkpoint inhibitors, could be a game changer for many otherwise lethal malignancies.”
CLIFFSIDE PARK, NJ, UNITED STATES, January 3, 2023 /EINPresswire.com/ -- One of the most common genetic changes resulting in malignancy is to the TP53 gene (which codes for the p53 protein)*. The normal “wild type” p53 protein inhibits cancer cell division and promotes cell death. However, many malignancies have mutations of the TP53 gene or inhibition of production of the normal p53. Through these events, normal cells can become malignant. — Neil Theise, MD
PT162 was designed to act as a p53-reactivating cell cycle checkpoint inhibitor, inducing cancer cell cycle arrest and/or apoptosis by restoring DNA-binding activity of mutant p53 protein.
Preliminary data demonstrates activity in castration resistant prostate cancer and multiple myeloma cell lines U266 and RPMI-8226. In all these tumors, restoration of normal p53 functions was confirmed by looking for the effects on other protein behaviors that follow on restored p53, such as cyclin D-1 (elevated in tumor cells, reduced after PT162 treatment) and p21 (reduced in tumor cells, increased to normal after treatment). These changes indicate a return to inhibition of cell division. Other restored gene activities showed increases in molecules that promote “programmed cell death” of malignant cells (e.g. Bax, BCL-2). Analysis of other downstream signaling molecules of the p53 pathway is ongoing.
Also, of note in the prostate cancer, expression of the NKX3.1 gene, a driver of this type of malignancy, was significantly diminished with PT162 treatment further supporting its function as an anti-cancer drug.
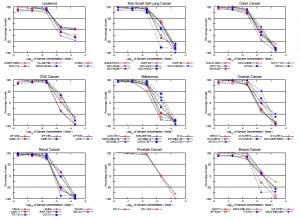
In addition PT162 was successfully tested in collaboration with the National Cancer Institute with activity demonstrated across multiple types of cancer.
The Palisades Therapeutics (PT) drug platform are patented molecules (composition of matter) of anti-cancer therapeutics that are expected to work in combination with other drugs to reduce or eliminate most cancers affected by p53.
Palisades Therapeutics invites industry leaders in the field of oncology such as Pfizer Inc. (NYSE: PFE); Bristol-Myers Squibb Company (NYSE: BMY); and Novartis AG (NYSE: NVS); to review our data.
National Cancer Institute (NCI) dose response curves for PT162 against multiple cancers are demonstrated on the graphs.
These data, produced by investigative colleagues of Palisades Therapeutics at a leading New York oncology research hospital, complementing the data found from an anti-cancer study performed by NCI, confirm the potency of PT162 as a modifier of cancer-associated changes in p53 gene function leading to significant anti-tumor pathway. p53 dysfunction is so common in a wide array of malignancies that compounds that could restore p53 functioning have been long sought in oncology. PT's Lead Scientist Dr. Neil Theise, Physician/Pathologist (NYU) believes that Palisades Therapeutics’ PT162 accomplishes that goal and, like recent discoveries of inflammatory checkpoint inhibitors, could be a game changer for many otherwise lethal malignancies.
*Su Y, Sai Y, Linfeng Z, et al. (2022) Current insights into the regulation of programmed cell death by TP53 mutation in cancer. Front Oncol. 12:1023427. doi: 10.3389
Randi Altschul
Pop Test Oncology/Palisades Therapeutics
+1 201-943-3770
randi@palisadestherapeutics.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.