Survey: COVID-19 vaccines tolerated well among at-risk patients
Inspire’s survey of thousands of patients with serious illnesses demonstrates safety of COVID-19 vaccines in vulnerable populations
The information our members share yields novel and dynamic perspectives, supporting improved care, scientific discoveries and better health outcomes.”
ARLINGTON, VIRGINIA, UNITED STATES, March 9, 2021 /EINPresswire.com/ -- People with serious medical conditions, including cancer, autoimmune disease and chronic lung conditions tolerate the Pfizer and Moderna COVID-19 vaccines well, according to new research from Inspire. — Brian Loew
“Cancer patients, autoimmune patients, lung patients, and others with serious comorbid conditions should not be worried about the vaccines, based on what we’re seeing in the survey,” said Stuart Goldberg, MD, an Inspire medical advisory board member, referring to the company’s HealthJourney COVID-19 research released today. “Findings from the many thousands of respondents who have already received COVID-19 vaccines and have not reported serious side effects demonstrate the relative safety of the COVID-19 vaccines in medically vulnerable populations.”
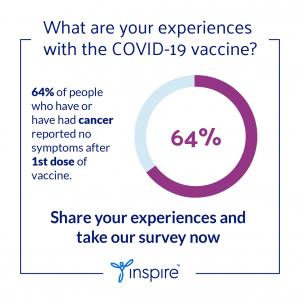
About 25%, or 5,600, of the survey participants with serious comorbid conditions reported receiving a COVID-19 vaccine. The self-reported local and systemic adverse events compared favorably with safety data from the clinical trials. Almost 30% reported none of the typical local side effects--pain, swelling, redness, itching--and nearly 60% stated no systemic side effects following first doses. For the second dose, around 20% had no side effects at the injection site and close to 30% experienced no follow-on side effects. No life-threatening events were reported.
Only 18% of respondents with chronic diseases expressed hesitancy in getting the vaccine, a lower percentage than general population opinion polls. Some worried that the vaccine was too new (50% of those who were hesitant) or were concerned that politics had played a role in its development (39%). However, a higher percentage of respondents compared to the US population had already received or planned to receive the vaccine, indicating a high level of vaccine acceptance among this population with increased COVID-19 mortality risk.
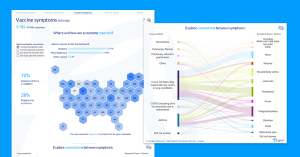
Inspire, the vital health community of more than two million patients and caregivers, has created visual tools to help explore the data with an interactive, filterable dashboard open to the public. There will be ongoing data collection over the following months to track the safety and efficacy profiles for those who receive the vaccine. Additional longitudinal data, including COVID-long haul symptoms, will be added to this dashboard.
“Our engaged members, who are patients and caregivers representing 3,300 health conditions with deep concentrations in cancer and rare disease, provide us with deep insights and perspectives about patients’ medical conditions and how they make decisions. In addition to participating in our COVID-19 survey, members of our community have supported and shared information with one another during this global pandemic,” said Inspire Founder/CEO Brian Loew. “The information our members share yields novel and dynamic perspectives, supporting improved care, scientific discoveries and better health outcomes.”
Inspire surveyed its online community of more than two million patients and caregivers, from over 100 countries. More than 26,000 patients and caregivers have responded so far to the longitudinal survey.
Inspire is opening the survey to the public and will launch additional follow-on surveys to track changes in vaccine perceptions. The continued research will also capture additional insights into symptoms and efficacy of the vaccines, and will examine Post-Acute Sequelae of SARS-CoV-2 infection (PASC), also known as Long COVID.
As part of its support for the many affected by the pandemic, Inspire also is a partner in the National COVID-19 Day project, developed by the Humanitarian Disaster Institute.
About Inspire
Inspire is the vital partner to life science companies, offering a unique resource for data and insights into patients’ rich and varied health journeys. Insights from Inspire's community of over two million members offer deep understanding of myriad conditions and their impact on patients. Visit us at inspire.com or corp.inspire.com.
John Novack
Inspire
+1 800-945-0381
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.