Implantable battery milestone toward Leviticus Cardio U.S. LVAD Trial
Leviticus Cardio embedded AlgoShield™ battery hazard detection to its implantable battery solution after positive concept review and feedback from the FDA.
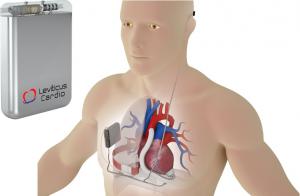
Leviticus Cardio's versatile transcutaneous Coplanar Energy Transfer (CET) for LVAD (Left Ventricular Assist Device) system was granted "Breakthrough Designation" status by the FDA in 2019. The Company’s unique LVAD battery solution offers an unprecedented combination of small size and exceptional internal battery capacity (up to 6 hours of wireless LVAD operation). Leviticus Cardio’s existing safety features and patents will be further strengthened with the addition of ALGOLION's proprietary, patented solution, to provide the next level of safety to meet regulatory and patient requirements.
ALGOLiON's AlgoShield advanced software provides early warning predictive intelligence algorithms to extend the lifetime of lithium-ion batteries while preventing safety hazards. Along with a robust coplanar energy transfer architecture, a unique battery solution represents a crucial piece in Leviticus’ patient-friendly, non-intrusive LVAD operation for fully unholstered daily activity.
Michael Zilbershlag, Leviticus Cardio's CEO, stated: "We are extremely excited to be embracing ALGOLiON’s AlgoShield to provide the greatest safety to patients and to meet regulatory requirements. The integrated platform will propel us another step closer to the U.S. EFS (Early Feasibility Study) commencement."
Niles Fleischer, Ph.D., ALGOLiON'S CEO, says, "We are delighted by this cooperation with Leviticus’ revolutionary wireless LVAD technology. It is yet another milestone for our company after we have already achieved numerous very significant business engagements in hazard mitigation of lithium-ion based batteries."
About Leviticus Cardio
Leviticus Cardio is a medical device company dedicated to improving the clinical outcome for patients with an implanted left ventricular assist device (LVAD) for the treatment of impaired cardiac function. Leviticus fully implanted wirelessly powered VAD system, represents groundbreaking innovation in contrast to contemporary driveline-based LVADs associated with a significant risk of infection and rehospitalizations. Also, FIVAD will incrementally improve the patient's quality of life by providing an almost normal lifestyle.
About ALGOLiON
ALGOLiON, is a privately-owned Israeli high-tech software company that developed early warning diagnostic algorithms designed to prevent lithium battery safety and fire hazards for various markets, including electric vehicles, portable electronics, medical devices, and aviation. The ALGOLiON technology is protected by a variety of patents globally. Its AlgoShield product has been successfully tested international certification labs TUV Sud in Germany and Impact Solutions in the U.K. The technology was recommended to the US FAA by Boeing for use in aviation. The software is running in an electric vehicle with a 43 kWh battery as a demonstration within the EU EVERLASTING H2020 project.
Michael Zilbershlag, CEO
Leviticus Cardio Ltd.
+972 3-629-2820
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Leviticus Cardio FIH
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.