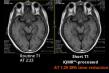
WindMIL Therapeutics Announces FDA Clearance of Investigational New Drug (IND) Application for Phase 2 Study of Marrow ...
BALTIMORE and PHILADELPHIA, June 25, 2019 (GLOBE NEWSWIRE) -- WindMIL Therapeutics, a clinical-stage company developing marrow-infiltrating lymphocytes (MILs™) for cancer immunotherapy, announced today that it has been cleared by the U.S. Food and Drug …