Angioplasty Balloons Market to Reach $3.6 Billion, Globally, by 2033 at 3.4% CAGR
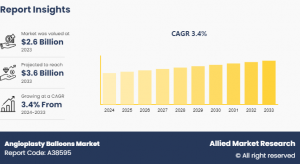
PORTLAND, GA, UNITED STATES, November 20, 2024 /EINPresswire.com/ -- The global angioplasty balloons market size was valued at $2.6 billion in 2023, and is projected to reach $3.6 billion by 2033, growing at a CAGR of 3.4% from 2024 to 2033. The global Angioplasty balloons market has experienced growth due to several factors such as increasing prevalence of cardiovascular diseases and advancements in balloon catheter technologies. Key factors include the rising aging population, lifestyle-related health issues, and a growing preference for minimally invasive procedures. In addition, innovations like drug-eluting balloons and improved catheter designs are enhancing market growth.
Angioplasty, often referred to as percutaneous transluminal coronary angioplasty (PTCA) , is a minimally invasive procedure utilized to open narrowed or blocked blood vessels that supply blood to the heart. The primary tool used in this procedure is the angioplasty balloon, a small balloon attached to a catheter. During angioplasty, a cardiologist inserts the catheter, typically through an artery in the groin or wrist, and guides it to the site of the blockage in the coronary artery. Once in position, the balloon is carefully inflated. This inflation compresses the plaque against the artery walls, effectively widening the vessel and restoring proper blood flow to the heart muscle. After the artery is sufficiently widened, the balloon is deflated and removed. Sometimes, a stent is placed at the site to keep the artery open. Angioplasty with balloon dilation has become a common and effective treatment for coronary artery disease, significantly reducing symptoms such as chest pain and improving the quality of life for patients with heart conditions.
Request Sample of the Report on: https://www.alliedmarketresearch.com/request-sample/A38595
Key Takeaways:
The angioplasty balloons industry study covers 20 countries. The research includes a segment analysis of each country in terms of value ($Million) for the projected period 2023-2033.
More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major angioplasty balloons industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions to achieve their most ambitious growth objectives.
Market Segmentation:
The angioplasty balloons market is segmented into type, application, end user, and region. On the basis of type, the market is classified into normal balloons, drug eluting balloons, cutting balloons, and scoring balloons. By application, the market is bifurcated into peripheral and coronary. By end user, the market is divided into ASCs, hospitals, and Cath labs. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
By Type:
Normal Balloons
Drug Eluting Balloons
Cutting Balloons
Scoring Balloons
By Application:
Peripheral
Coronary
By End User:
ASCs
Hospitals
Cath Labs
By Region:
North America (U.S., Canada, Mexico)
Europe (France, Germany, Italy, Spain, UK, Rest of Europe)
Asia-Pacific (China, Japan, India, South Korea, Australia, Rest of Asia-Pacific)
LAMEA (Brazil, South Africa, Saudi Arabia, Rest of LAMEA)
Want to Explore More, Connect to our Analyst - https://www.alliedmarketresearch.com/connect-to-analyst/A38595
Recent Developments:
In January 2022, Cardiovascular Systems, Inc. and OrbusNeich Medical Company Ltd. announced that the U.S. Food and Drug Administration (FDA) had provided approval to the Scoreflex NC, a focused force Percutaneous Transluminal Coronary Angioplasty (PTCA) scoring balloon consisting of a dual-wire system, which facilitates controlled plaque modification at low pressure.
In February 2023, SIS Medical AG launched the OPN NC PTCA dilatation catheter in the U.S. The devices, which uses the TWIN-Wall technology, has been approved by the FDA and provides high-pressure resistance of up to 35atm.
In November 2022, Johnson & Johnson announced a definitive agreement for the acquisition of Abiomed, a leader in heart support and recovery technologies, with an upfront payment of USD 380 per share, which equates to USD 16.6 billion. With this acquisition, Johnson & Johnson aims to develop breakthrough treatments for cardiovascular diseases and reach more patients globally.
Competitive Landscape:
Abbott Laboratories
B. Braun Melsungen AG
BIOTRONIK SE & Co. KG
Boston Scientific Corporation
C. R. Bard, Inc.
Cardinal Health, Inc.
Medtronic plc.
MicroPort Scientific Corporation
Terumo Corporation
Key Benefits For Stakeholders:
This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the angioplasty balloons market analysis from 2024 to 2033 to identify the prevailing angioplasty balloons market opportunities.
The market research is offered along with information related to key drivers, restraints, and opportunities.
Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
In-depth analysis of the angioplasty balloons market segmentation assists to determine the prevailing market opportunities.
Major countries in each region are mapped according to their revenue contribution to the global market.
Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
The report includes the analysis of the regional as well as global angioplasty balloons market trends, key players, market segments, application areas, and market growth strategies.
For Purchase Related Queries/Inquiry - https://www.alliedmarketresearch.com/purchase-enquiry/A38595
About Allied Market Research:
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Wilmington, Delaware. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domains. AMR offers its services across 11 industry verticals including Life Sciences, Consumer Goods, Materials & Chemicals, Construction & Manufacturing, Food & Beverages, Energy & Power, Semiconductor & Electronics, Automotive & Transportation, ICT & Media, Aerospace & Defense, and BFSI.
We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Allied Market Research CEO Pawan Kumar is instrumental in inspiring and encouraging everyone associated with the company to maintain high quality of data and help clients in every way possible to achieve success. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.

Angioplasty, often referred to as percutaneous transluminal coronary angioplasty (PTCA) , is a minimally invasive procedure utilized to open narrowed or blocked blood vessels that supply blood to the heart. The primary tool used in this procedure is the angioplasty balloon, a small balloon attached to a catheter. During angioplasty, a cardiologist inserts the catheter, typically through an artery in the groin or wrist, and guides it to the site of the blockage in the coronary artery. Once in position, the balloon is carefully inflated. This inflation compresses the plaque against the artery walls, effectively widening the vessel and restoring proper blood flow to the heart muscle. After the artery is sufficiently widened, the balloon is deflated and removed. Sometimes, a stent is placed at the site to keep the artery open. Angioplasty with balloon dilation has become a common and effective treatment for coronary artery disease, significantly reducing symptoms such as chest pain and improving the quality of life for patients with heart conditions.
Request Sample of the Report on: https://www.alliedmarketresearch.com/request-sample/A38595
Key Takeaways:
The angioplasty balloons industry study covers 20 countries. The research includes a segment analysis of each country in terms of value ($Million) for the projected period 2023-2033.
More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major angioplasty balloons industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions to achieve their most ambitious growth objectives.
Market Segmentation:
The angioplasty balloons market is segmented into type, application, end user, and region. On the basis of type, the market is classified into normal balloons, drug eluting balloons, cutting balloons, and scoring balloons. By application, the market is bifurcated into peripheral and coronary. By end user, the market is divided into ASCs, hospitals, and Cath labs. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
By Type:
Normal Balloons
Drug Eluting Balloons
Cutting Balloons
Scoring Balloons
By Application:
Peripheral
Coronary
By End User:
ASCs
Hospitals
Cath Labs
By Region:
North America (U.S., Canada, Mexico)
Europe (France, Germany, Italy, Spain, UK, Rest of Europe)
Asia-Pacific (China, Japan, India, South Korea, Australia, Rest of Asia-Pacific)
LAMEA (Brazil, South Africa, Saudi Arabia, Rest of LAMEA)
Want to Explore More, Connect to our Analyst - https://www.alliedmarketresearch.com/connect-to-analyst/A38595
Recent Developments:
In January 2022, Cardiovascular Systems, Inc. and OrbusNeich Medical Company Ltd. announced that the U.S. Food and Drug Administration (FDA) had provided approval to the Scoreflex NC, a focused force Percutaneous Transluminal Coronary Angioplasty (PTCA) scoring balloon consisting of a dual-wire system, which facilitates controlled plaque modification at low pressure.
In February 2023, SIS Medical AG launched the OPN NC PTCA dilatation catheter in the U.S. The devices, which uses the TWIN-Wall technology, has been approved by the FDA and provides high-pressure resistance of up to 35atm.
In November 2022, Johnson & Johnson announced a definitive agreement for the acquisition of Abiomed, a leader in heart support and recovery technologies, with an upfront payment of USD 380 per share, which equates to USD 16.6 billion. With this acquisition, Johnson & Johnson aims to develop breakthrough treatments for cardiovascular diseases and reach more patients globally.
Competitive Landscape:
Abbott Laboratories
B. Braun Melsungen AG
BIOTRONIK SE & Co. KG
Boston Scientific Corporation
C. R. Bard, Inc.
Cardinal Health, Inc.
Medtronic plc.
MicroPort Scientific Corporation
Terumo Corporation
Key Benefits For Stakeholders:
This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the angioplasty balloons market analysis from 2024 to 2033 to identify the prevailing angioplasty balloons market opportunities.
The market research is offered along with information related to key drivers, restraints, and opportunities.
Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
In-depth analysis of the angioplasty balloons market segmentation assists to determine the prevailing market opportunities.
Major countries in each region are mapped according to their revenue contribution to the global market.
Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
The report includes the analysis of the regional as well as global angioplasty balloons market trends, key players, market segments, application areas, and market growth strategies.
For Purchase Related Queries/Inquiry - https://www.alliedmarketresearch.com/purchase-enquiry/A38595
About Allied Market Research:
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Wilmington, Delaware. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domains. AMR offers its services across 11 industry verticals including Life Sciences, Consumer Goods, Materials & Chemicals, Construction & Manufacturing, Food & Beverages, Energy & Power, Semiconductor & Electronics, Automotive & Transportation, ICT & Media, Aerospace & Defense, and BFSI.
We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Allied Market Research CEO Pawan Kumar is instrumental in inspiring and encouraging everyone associated with the company to maintain high quality of data and help clients in every way possible to achieve success. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
David Correa
Allied Market Research
800-792-5285
email us here
Visit us on social media:
Facebook
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.