Recce Pharmaceuticals Announces Positive Preclinical Efficacy Data Against WHO Priority Pathogen Acinetobacter baumannii
-
RECCE® 327 (R327) demonstrated significant bactericidal activity against ‘superbug’ Acinetobacter baumannii (A. baumannii) demonstrating 99.99999% log reduction (>7.5 log reduction) in adult human epidermal ‘skin’ cells
- A. baumannii is most commonly responsible for urinary tract infections, ventilator-associated pneumonia, central line-associated bloodstream infections, persistent wound infections and meningitis
-
The data supports upcoming Phase II Acute Bacterial Skin and Skin Structure Infection (ABSSSI) trials as A. baumannii infections are notoriously difficult to treat, especially in their drug resistant ‘superbug’ form
SYDNEY, July 10, 2024 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Limited (ASX:RCE, FSE:R9Q), (the Company), the Company developing a new class of synthetic anti-infectives, is pleased to report promising results from its latest study on the efficacy of RECCE® 327 (R327) against multidrug- resistant (MDR) World Health Organization (WHO) priority pathogen Acinetobacter baumannii (A. baumannii). The study was conducted at Recce’s Anti-Infective Research (AIR) unit within Murdoch Children’s Research Institute.
“These outstanding results highlight the potent efficacy of R327 in combating MDR bacteria, a significant global health challenge,” commented James Graham, CEO of Recce Pharmaceuticals. “The ability of R327 to achieve such substantial reductions in bacterial load and maintain its effectiveness over 24 hours is a testament to its potential as a leading anti-infective treatment. We are excited about these findings and their implications for the future of infection management.”
Dr. Sohinee Sarkar, a leading researcher at Murdoch Children’s Research Institute, added, “The significant bactericidal activity observed with R327, particularly its ability to reduce bacterial presence to below the limit of detection, marks a major advancement in our fight against resistant pathogens. The sustained efficacy of R327 compared to ciprofloxacin underscores its potential as a superior treatment option. These results are promising and pave the way for further development and application of R327 in clinical settings.”
The study demonstrated R327’s bactericidal activity when compared to placebo and ciprofloxacin in 1-hour post treatment and at 24-hours post treatment in primary human epidermal keratinocytes (skin cells) infected with A. baumannii.
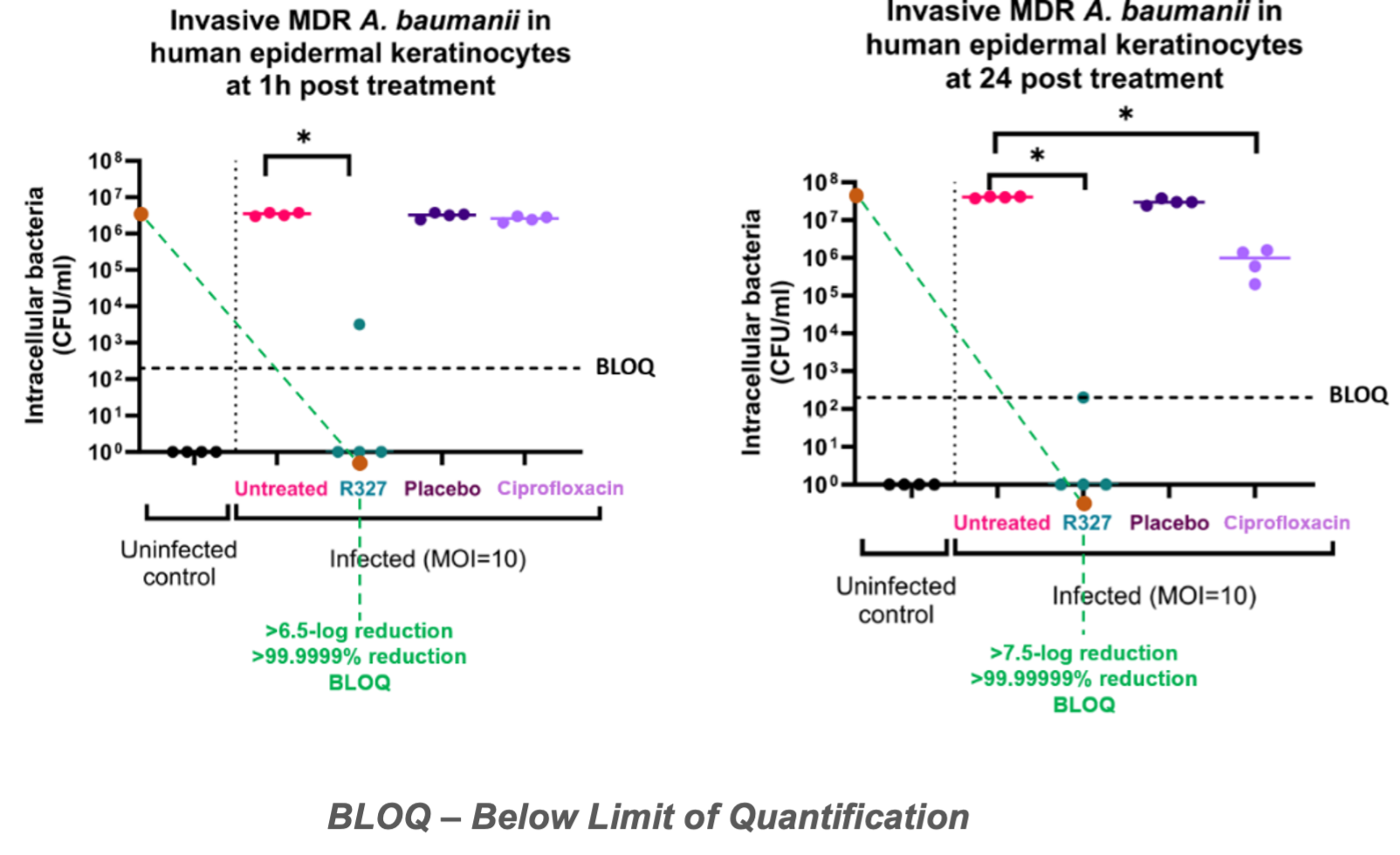
Key Findings from the study include:
- 1-hour Post-treatment Efficacy: Within the first hour post-treatment of invasive (intracellular) multidrug-resistant A. baumannii in primary human epidermal keratinocytes, R327 achieved a >6.5 log reduction, rendering the bacteria below the limit of quantification (BLOQ). This demonstrates the rapid and effective bactericidal action of R327. In contrast, ciprofloxacin did not show any reduction in intracellular bacterial burden at this early timepoint.
- 24-hour Sustained Efficacy: R327 maintained its efficacy at >6.5 log or >99.9999% reduction in intracellular bacteria even after 24 hours, a critical factor in infection management. In comparison, ciprofloxacin treatment only resulted in ~1 log reduction in bacterial numbers over the same period.
The WHO has listed A. baumannii as one of the top priority pathogens due to its high levels of resistance to multiple antibiotics. A. baumannii is now a significant global health threat, known for causing severe infections, both in hospital settings and within the community.
Prevalent infections from A. baumannii include persistent wound infections, central line-associated bloodstream infections (BSIs), catheter-associated Urinary Tract Infections (UTIs), ventilator-associated pneumonia (VAP), and meningitis. Infections caused by MDR A. baumannii can lead to prolonged hospital stays, increased healthcare costs, and high mortality rates, with some studies reporting mortality rates as high as 50% in critically ill patients.
The results from this study build upon successful Phase II Diabetic Foot Ulcer Infection data including recent completion and positive data from the Phase I/II rapid infusion clinical trial, with R327 demonstrating safety and efficacy against E. coli in ex vivo testing. The demonstrated efficacy against MDR A. baumannii further builds out the efficacy profile of R327 against UTI-causing pathogens and will support the initiation of a Phase II trial in patients with UTI/Urosepsis.
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) is developing a New Class of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic-resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline includes three patented, broad-spectrum, synthetic polymer anti-infectives: RECCE® 327 (R327) as an intravenous and topical therapy that is being developed for the treatment of serious and potentially life-threatening infections due to Gram-positive and Gram-negative bacteria, including their superbug forms; RECCE® 435 (R435) as an orally administered therapy for bacterial infections; and RECCE® 529 (R529) for viral infections. Through their multi-layered mechanisms of action, Recce’s anti-infectives have the potential to overcome the processes utilised by bacteria and viruses to overcome resistance – a current challenge facing existing antibiotics.
The World Health Organization (WHO) added R327, R435, and R529 to its list of antibacterial products in clinical development for priority pathogens, recognising Recce’s efforts to combat antimicrobial resistance. The FDA granted R327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act, providing Fast Track Designation and 10 years of market exclusivity post approval. R327 is also included on The Pew Charitable Trusts’ Global New Antibiotics in Development Pipeline as the sole synthetic polymer and sepsis drug candidate in development.
Recce wholly owns its automated manufacturing, supporting current clinical trials. Recce’s anti-infective pipeline aims to address synergistic, unmet medical needs by leveraging its unique technologies.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 9256 2571
James.graham@recce.com.au
Media & Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
ageddes@citypublicrelations.com.au
Media (USA)
Michael Fitzhugh
LifeSci Communications
mfitzhugh@lifescicomms.com
Investor Relations (USA & EU)
Guillame van Renterghem
LifeSci Advisors
gvanrenterghem@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/de42834e-6ecd-4cbb-ae4c-c4a7cbdb1dbd

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.



