Study reveals that our ancient single cell ancestors fought off giant viruses with Z-DNA and Z-RNA
The role of the left-handed Z-DNA and Z-RNA conformation in human immunity has been long overlooked due to an over reliance on fly and worm genetic models
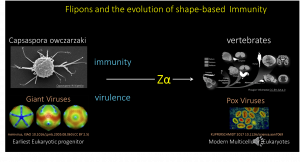
The large sets of DNA data generated by genome-sequencing projects around the world allowed tracking of the Zα domain back to the earliest known single cell organism that is thought to be the ancient precursor of multicellular organisms like humans. Interestingly, the Zα domain is associated with the same protein, ADAR1, that protects the modern-day descendants against threats to this day. More intriguing is that the ancient giant mimi- and pithoviruses also have Zα domains to modulate immune response against them, just like their modern-day descendants like smallpox that cause pandemics and other human disease.
The Z-RNAs that regulate the Zα-dependent innate immune responses are encoded by host genomes. The cell monitors expression levels of these Z-RNAs to determine how well it is doing. Increased expression of Z-RNA indicates either a viral infection or that the cell has become dysfunctional. When expressed at high levels, the left-handed Z-RNAs activate inflammatory and programmed cell death pathways. The system is introspective as it is based on the cell’s perception of its internal status, rather than relying on the direct sensing of events external to the cell.
The Z-RNAs are mostly encoded by repeat sequences that comprise over half of the human genome. These sequences are called flipons as they can adopt multiple conformations in a cell without alterations to their sequence or any breakage of any DNA strands. Most frequently the flipons lie within the long and short, interspersed, nuclear elements, commonly known as LINE and SINEs. As B-DNA, the repeat elements are so frequent that they are uninformative. Only by changing their shape, do they provide information. They act as binary switches, as flipons can only be in one state or another. By changing shape, they enable cells to program context-specific immune responses.
Initially the retroelements attacked by copying their RNAs back into the genome at multiple sites. They were an existential threat that has now been tamed. Many of their sequences are components of normal host transcripts. The Z-RNA they form now helps the host identify them as self and distinguishes them from viral RNAs. Only when their expression is induced at high levels does the immune response kick in. That this system is so ancient gives proof of its effectiveness.
To prevent the spread of retroelements through the genome, there was strong selection against the enzymes that copied retroelement RNAs back into DNA. This precluded the use of the immune strategies evolved by other organisms, such as those used in C. elegans and D. melanogaster. These alternative mechanisms rely on RNA-dependent polymerases to amplify RNAs that specifically target viral transcripts and interfere with their function. In contrast to the human response that is based on the Z-RNA encoded by the host genome, RNA interference depends on the viral genome. The human Z-RNA -based system provides protection against both old and newly emerging viruses. It is available immediately on infection. The shape-based response also protects against cancerous cells when promiscuous transcription of the genome within tumors lead to high levels of Z-RNA and Z-DNA expression by retroelements.
The paper opens up new understanding of differences between humans and the model genetic organisms used to study innate immunity. Already therapies are in development that target flipon-based immune responses. The paper also generates new hypotheses to explore experimentally.
About InsideOutBio: InsideOutBio is a start-up focused on developing a novel class of proprietary therapeutics to 'light' up tumors for the immune system to kill by reprogramming self/nonself pathways within cancer cells. Dr. Herbert leads discovery at InsideOutBio. His work on Z-DNA was foundational to the discovery of flipons. These statements about InsideOutBio comply with Safe-Harbor laws. They are forward-looking and involve known and unknown risks and uncertainties. They are not guarantees of future performance and undue reliance should not be placed on them.
Alan Herbert
InsideOutBio, Inc
+1 617-584-0360
email us here
Visit us on social media:
X
LinkedIn
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.