PDS Biotech Provides Data Update from Ongoing VERSATILE-002 Phase 2 Clinical Trial in Head and Neck Cancer
Majority of patients continue to be followed for survival with multiple patients approaching 3 years
Median Overall Survival (mOS) remains at 30 months based on the most recent data cut with approximately six months of additional follow-up
PRINCETON, N.J., June 12, 2024 (GLOBE NEWSWIRE) -- PDS Biotechnology Corporation (Nasdaq: PDSB) (“PDS Biotech” or the “Company”), a late-stage immunotherapy company focused on transforming how the immune system targets and kills cancers and the development of infectious disease vaccines, today provided a data update from its ongoing VERSATILE-002 Phase 2 clinical trial. VERSATILE-002 is evaluating Versamune® HPV + KEYTRUDA® (pembrolizumab) in patients with HPV16-positive head and neck squamous cell cancer (“HNSCC”).
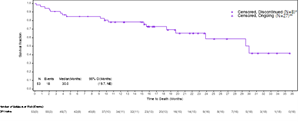
The Kaplan-Meier analysis described below captures the survival data for immune checkpoint inhibitor (“ICI”) naïve patients from the ongoing VERSATILE-002 Phase 2 clinical trial. All patients whose data are reported in the Kaplan-Meier analysis are properly censored to confirm their follow-up and survival status.
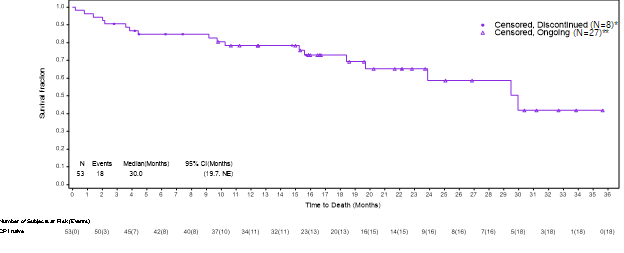
Based on a data cut as of May 17, 2024, the updated survival data for the cohort of ICI naïve patients after an additional follow-up of approximately 6 months in the VERSATILE-02 Phase 2 clinical trial with a total of 53 enrolled patients was as follows:
- mOS is 30 months, consistent with data presented at the Company’s Key Opinion Leader event on May 9, 2024, which was based on a data cut as of November 30, 2023.
- 27 of the censored patients remained alive and were awaiting their next clinical assessment, 6 censored patients had withdrawn consent for further follow-up, and 2 patients had been lost to follow-up, and 18 patients had died.
- The lower limit of the 95% confidence interval is 19.7 months, and the upper limit is not yet estimable, as the majority of the patients continue to be followed for survival.
Full data from the May 17, 2024 data cut are expected to be announced in Q3 2024.
Dr. Kirk Shepard, M.D., Chief Medical Officer of PDS Biotech stated, “In recurrent and/or metastatic HNSCC, objective response rate and progression-free survival have generally not translated into increased survival, and under current standards of care survival rates are well established to be less than 18 months. We believe that our VERSATILE-002 clinical trial and triple combination trial provide us with the critical survival information needed to effectively design the statistical primary endpoint in our planned Phase 3 trial.”
The Company continues to advance its clinical strategy, which consists of a three arm registrational trial in first line treatment of HPV16-positive recurrent/metastatic HNSCC. The planned trial has two active arms: the double combination of Versamune® HPV + pembrolizumab, and the triple combination of Versamune® HPV + PDS01ADC + pembrolizumab. PDS01ADC is the Company’s tumor-targeted IL-12-fused antibody-drug conjugate (“ADC”), which has shown promise in ongoing Phase 2 clinical trials including a Phase 2 clinical trial of Versamune® HPV + PDS01ADC + an investigational ICI conducted by the National Cancer Institute.
About PDS Biotechnology
PDS Biotechnology is a late-stage immunotherapy company focused on transforming how the immune system targets and kills cancers and the development of infectious disease vaccines. The Company plans to initiate a pivotal clinical trial in 2024 to advance its lead program in advanced head and neck squamous cell cancers. PDS Biotech’s lead program is a proprietary dual-acting combination of IL-12 fused antibody drug conjugate (ADC) PDS01ADC and T-cell activator Versamune® HPV in regimen with a standard-of-care immune checkpoint inhibitor. We believe that proof-of-concept long-term data have shown positive survival results and tumor shrinkage with this combination and indicate favorable tolerability.
We believe that with a novel investigational “inside-outside” mechanism, the PDS01ADC and Versamune® HPV immunotherapy has shown compelling results with potential to successfully disrupt a tumor’s inside defenses, while also generating potent, targeted killer T-cells to attack the tumor from the outside. We also believe that data from more than 350 patients, as well as ongoing clinical trials across multiple tumor types and standard treatment regimens, have validated the potential for both platforms and point to potential broad utility.
Forward Looking Statements
This communication contains forward-looking statements (including within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended, and Section 27A of the United States Securities Act of 1933, as amended) concerning PDS Biotechnology Corporation (the “Company”) and other matters. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the Company’s management, as well as assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” “forecast,” “guidance”, “outlook” and other similar expressions among others. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the Company’s ability to protect its intellectual property rights; the Company’s anticipated capital requirements, including the Company’s anticipated cash runway and the Company’s current expectations regarding its plans for future equity financings; the Company’s dependence on additional financing to fund its operations and complete the development and commercialization of its product candidates, and the risks that raising such additional capital may restrict the Company’s operations or require the Company to relinquish rights to the Company’s technologies or product candidates; the Company’s limited operating history in the Company’s current line of business, which makes it difficult to evaluate the Company’s prospects, the Company’s business plan or the likelihood of the Company’s successful implementation of such business plan; the timing for the Company or its partners to initiate the planned clinical trials for PDS01ADC, PDS0101, PDS0203 and other Versamune® and Infectimune® based product candidates; the future success of such trials; the successful implementation of the Company’s research and development programs and collaborations, including any collaboration studies concerning PDS01ADC, PDS0101, PDS0203 and other Versamune® and Infectimune® based product candidates and the Company’s interpretation of the results and findings of such programs and collaborations and whether such results are sufficient to support the future success of the Company’s product candidates; the success, timing and cost of the Company’s ongoing clinical trials and anticipated clinical trials for the Company’s current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including the Company’s ability to fully fund its disclosed clinical trials, which assumes no material changes to the Company’s currently projected expenses), futility analyses, presentations at conferences and data reported in an abstract, and receipt of interim or preliminary results (including, without limitation, any preclinical results or data), which are not necessarily indicative of the final results of the Company’s ongoing clinical trials; any Company statements about its understanding of product candidates mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs and any collaboration studies; the Company’s ability to continue as a going concern; and other factors, including legislative, regulatory, political and economic developments not within the Company’s control. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the other risks, uncertainties, and other factors described under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in the documents we file with the U.S. Securities and Exchange Commission. The forward-looking statements are made only as of the date of this press release and, except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Versamune® and Infectimune® are registered trademarks of PDS Biotechnology Corporation.
Keytruda® is a registered trademark of Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, N.J., USA.
Investor Contact:
Mike Moyer
LifeSci Advisors
Phone +1 (617) 308-4306
Email: mmoyer@lifesciadvisors.com
Media Contact:
Gina Mangiaracina
6 Degrees
Phone +1 (917) 797-7904
Email: gmangiaracina@6degreespr.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a877ddf8-ce6d-4333-9316-38fe88c1009a

The Kaplan-Meier analysis described below captures the survival data for immune checkpoint inhibitor (“ICI”) naïve patients from the ongoing VERSATILE-002 Phase 2 clinical trial.
The Kaplan-Meier analysis described below captures the survival data for immune checkpoint inhibitor (“ICI”) naïve patients from the ongoing VERSATILE-002 Phase 2 clinical trial. All patients whose data are reported in the Kaplan-Meier analysis are properly censored to confirm their follow-up and survival status.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.