Flipons are tiny DNA actuators that play a previously unknown role in gene expression
Everything you wanted to know about the role of flipons in DNA transcription but were afraid to ask
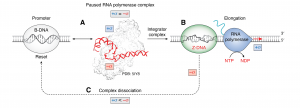
That Z-DNA does play a role was suggested by many different sets of evidence where chemical probing showed the flip from B- to Z-DNA as genes were transcribed. The energy to power Z-DNA formation comes from the RNA polymerase as it unwinds DNA to make a gene transcript. By convention, this underwinding of DNA is called negative supercoiling. However, the effect of Z-DNA formation on the action of RNA polymerase was unknown. The search for proteins that by bound to specific flipon sequences in the Z-DNA conformation has not so far found any such examples. The idea was that such proteins could act as transcription factors to promote RNA polymerase engagement. Other models were proposed, suggesting that Z-DNA was there to localize enzymes like topoisomerases to stop Z-DNA formation by sequences that had other roles in transcription that depended on them being in the B-DNA conformation. There is some support for this idea and also evidence against. Others decided not to consider the problem at all. They though that working with high energy DNA conformations like Z-DNA was experimentally difficult, noting that many of the initial findings were later found to be artefactual.
The new work starts simply with the observation that to start transcription, it is necessary to pry the B-DNA helix open so that the RNA polymerase can dock. To compensate for the negative supercoiling required, DNA must overwind in the opposite direction. Proteins in the pre-initiation complex (PIC) accomplish the unwinding by promoting positive supercoiling of the DNA. Indeed, the stability of PIC depends on positive supercoiling of the DNA. Here is where Z-DNA comes in. By capturing the extra energy generated by the RNA polymerase transcribing away from the promoter, enough negative supercoiling is accumulated by Z-flipons to overcome the positive supercoiling necessary to stabilize the PIC. At some point, the PIC pops off the promoter. The reset then enables the next round of transcription to begin. The flipon is then acting as an actuator to ensure that promoters turn over. They serve as exquisitely small mechanical devices to enable the transcription cycle of load, fire and reset. Flipons make this process sensitive to the cellular context as their propensity to flip can be tuned in response to environmental stresses.
The findings in the study have implications for how transcription is regulated. The stability of the PIC affects turnover rate. Interestingly, the PIC composition is highly variable, meaning that some promoters are faster to reset than others. As a result, higher transcription rates are possible for genes where the PIC pops off easily. The paper also explores how the flip B-DNA to Z-DNA depends on flipon sequence and on various DNA modifications. Other factors such as small RNAs that bind to the single-stranded regions formed as flipons change from one conformation to another can alter the complexes that assemble at the promoter. The different complexes assembled offer an explanation for the observed asymmetry of epigenetic marks that depend on the direction of transcription form the promoter.
The therapeutic implications of these findings are discussed by Dr. Herbert. In particular, parameters for the design of small RNAs are given for upregulating gene expression and offset the loss of transcripts seen in many diseases.
InsideOutBio is a start-up focused on developing a novel class of proprietary therapeutics to ‘light’ up tumors for the immune system to kill by reprogramming self/nonself pathways within cancer cells. Dr. Herbert leads discovery at InsideOutBio. These statements about InsideOutBio comply with Safe-Harbor laws. They are forward-looking and involve known and unknown risks and uncertainties. They are not guarantees of future performance and undue reliance should not be placed on them.
Alan Herbert
InsideOutBio, Inc
+1 617-584-0360
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.