Flipons help ADAR1 turn off inflammation
More evidence for a role of Z-RNA in human disease
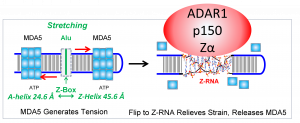
CHARLESTOWN, MA, UNITED STATES, July 16, 2023/EINPresswire.com/ -- In a paper just published in Cell Reports, evidence id produced that flipons suppress interferon responses by the double-stranded RNA editing enzyme ADAR1, even when the enzyme is fully catalytically active. Although the field long resisted a role for Z-RNA in regulating ADAR1 activity, the evidence continues to show that sequences capable of adopting alternative conformations, called flipons, do indeed play an important role in the suppression of immune responses by ADAR1, even when the enzyme has normal catalytic function.Each cell contains a number of sensor proteins for the nucleic acids produced during viral infection. Amongst these are those are MDA5 that responds to double-stranded RNA (dsRNA). However, the cell must avoid responding to dsRNA produced normally. The ADAR1 enzyme provides the necessary protection. An interferon induced p150 form of ADAR1 suppresses responses to host transcripts. How the enzyme does this has been a long—standing question. A role of the Z-RNA domain in ADAR1 p150 was once controversial, but now has been supported by a number of genetic and biological studies. In particular, Z-RNA formed by repetitive elements embedded within host RNA appears central to negatively regulating the response against self dsRNA. Still it remained uncertain as to whether Z-RNA formation disrupts dsRNA by editing of one or other RNA strand so they no longer pair, or whether Z-RNA is involved in some other process.
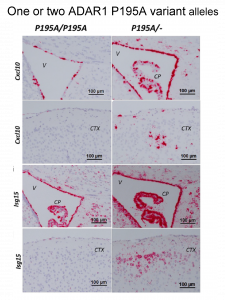
Recent papers have produced conflicting results as to how Z-RNA affects editing. The Cell Reports paper resolves the controversy. All papers study an ADAR1 P193A variant found commonly in 0.3% of humans and causative for disease when paired with n ADAR1 p150 null allele (i.e. it produces no protein). The authors independently produced mouse models studying with an ADAR1 P195A variant (equivalent to human P193A). The teams all report an activation of interferons stimulated genes due to the loss of function ADAR1 P195A variant, even though the enzyme activity was normal.
The current study uses a mouse model where the amplification of interferon responses is prevented by deletion of the MDA5 protein. The induction of interferon stimulated genes depended on the number of copies pf the P195A allele present rather than the general level of editing, or on editing of any one of the well characterized p150 substrates that recode a protein sequence by changing adenosine to inosine. The difference in results from a previous study appears due to technical reasons. In the study that the other groups failed to reproduce, the mouse mutations used were not sufficiently characterized and confounded the analysis.
The current paper does not fully recapitulate human disease caused by the human P193A variant, but does show changes in the brain that resemble those seen in patients. One possible explanation is that by binding Z-RNA, ADAR1 inhibits activation of the pro-inflammatory Z-RNA and Z-DNA binding protein ZBP1. ZBP1 like ADAR1 -150 is also interferon induced and further work will address the role of the interaction between the two proteins in human disease.
The work was performed by Qingde Wang and his group at the University of Pittsburgh School of Medicine in collaboration with Alan Herbert at InsideOutBio
Alan Herbert
InsideOutBio
+1 617-800-7531
email us here
Visit us on social media:
Twitter
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.