Medtronic Leads the Way: Insights into the European Peripheral Vascular Device Market
VANCOUVER, BRITISH COLUMBIA, CANADA, June 12, 2023/EINPresswire.com/ -- iData Research, a global consulting and market research firm, has just released exclusive research on the European Peripheral Vascular Devices Market. The research provides valuable insights into market trends, developments, and competition. An emerging trend in the peripheral vascular market is the adoption of cutting-edge technologies and minimally invasive techniques for peripheral vascular procedures. These advanced technologies encompass catheter-based treatments and devices, including drug-coated balloons, atherectomy devices, and stent grafts. The incorporation of these innovative technologies brings numerous advantages compared to conventional surgical procedures, such as shorter recovery periods, reduced patient discomfort, and enhanced clinical outcomes. With ongoing investments from medical device companies and healthcare providers, the Peripheral Vascular Device Market is poised to further evolve, placing greater importance on patient outcomes, cost-effectiveness, and innovation.
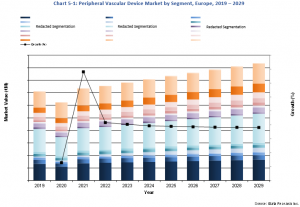
According to iData's 2023 European Market Report for Peripheral Vascular Devices, the market reached a valuation of just over €1.5 billion in 2022 and is projected to experience a steady growth rate throughout the forecast period, reaching a value of almost €1.9 billion in 2029. This report suite includes unit sales, average selling prices, market drivers and limiters, competitive market share analysis, and more.
iData's analysis also includes detailed segmentation on an astonishing 18 market segments within the European Peripheral Vascular Devices Market. Some notable segments include the peripheral vascular stent market, CTO device market, stent graft market, transcatheter embolization market, vascular closure device market, introducer sheath market and much more.
Medtronic, the market leader in Europe's Peripheral Vascular Market in 2022, maintained its position through a strong presence in the stent graft market and a diverse product portfolio. Key offerings such as the Admiral IN.PACT™ drug-coated balloons and Endurant™ and Valiant™ stent grafts contributed significantly to the company's revenue. Cook Medical secured the second spot by excelling in the stent graft market and leading the inferior vena cava filter (IVCF) market. Their Zenith® family of stent grafts stood out as the company's flagship product. Boston Scientific, initially the second-leading competitor after merging with BTG, slipped to third place due to market share loss to Cook Medical. Nevertheless, Boston Scientific made impressive advancements in venous treatment and transcatheter embolization, establishing a leading position in both markets. With a complete portfolio for treating venous diseases, Boston Scientific is poised to gain market share in the European Peripheral Vascular Market.
Key Questions Answered in this Report:
How has the European Peripheral Vascular Device Market performed so far and how will it perform in the coming years?
What are the key regional markets?
What are the key driving factors and challenges in the industry?
What is the structure of the European Peripheral Vascular Device Market and what are the market shares of the key players?
What is the degree of competition in the industry?
Follow the link below to download a Free Research Summary of the European Peripheral Vascular Device Report:
https://idataresearch.com/product/peripheral-vascular-devices-market-europe/
For Further Information
More insights like this can be found in the latest reports by iData. Please email us at info@idataresearch.net or register online for a brochure and synopsis.
About iData
iData Research is an international consulting and market research firm dedicated to empowering confident strategic decisions within the medical device, dental, and pharmaceutical industries.
www.idataresearch.com
Dejan Popic
iData Research
+1 604-266-6933
email us here
Visit us on social media:
Facebook
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.