Arowor Corp. Launched to Develop Biopharmaceuticals for Rare Infant Disorders and Disaster Response Plans

Corporate Photo of Aron Workman, CEO - Arowor Corp.
Arowor Corp. launched on New Year’s Day, 2023. For more information, visit its website at https://arowor.com and email <contact@arowor.com>.
UNITED STATES, January 5, 2023 /EINPresswire.com/ -- Arowor Corp. was recently incorporated in Delaware, effective January 1, 2023, 5:00 AM ET. The incorporator and initial director is Aron Workman, Chairman and CEO. For more information, visit the Arowor Corp. website at https://arowor.com and email <contact@arowor.com>.
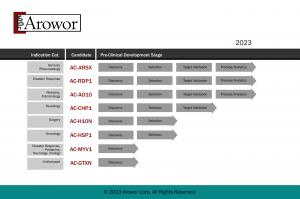
Arowor Corp. develops biopharmaceuticals for rare infant disorders and disaster response plans. The Arowor Corp. pipeline is currently comprised of over one dozen candidates in various phases of laboratory and pre-clinical stage development, including AC-AO10, AC-H10N, AC-MYV1, and AC-RDP1:
• AC-AO10 is a protein therapeutic under development for chronic lung disease (CLD) in neonates.
• AC-H10N is a peptide therapeutic under development for perioperative usage across surgery.
• AC-MYV1 is a vector therapeutic under development for acute flaccid myelitis (AFM) in infants.
• AC-RDP1 is a protein therapeutic under development for ARS/DEARE from nuclear fallout.
The pharmaceutical-military-industrial complex has suffered what could be a catabolic overhaul due to the coronavirus epidemic. The epidemic demonstrated that disaster response drugs no longer need be small powders selected primarily for their manufacturability and storage criteria.
“The pharmaceutical industry has complex structure; military-industrial projects too require multidisciplinary attention. In applied biochemistry, structure and function are commutative. We approach that 2x2 complex wherein disaster response drug development resides – first and foremost with an awe and reverence for life on Earth.” – Aron Workman, CEO
Rising international nuclear tension and turmoil are expected to amplify prior to 2026. Radioprotectant development is an ongoing concern for commercial and governmental drug development entities.
Arowor Corp. is capitalizing on rational drug design and discovery methodologies which include its HPC, MPM, and SODA platforms. The Arowor Corp. high-performance computing (HPC) platform yields simulations, studies, and analytics, and its non-naturally occurring analog portfolio is predominantly comprised of codon-restricted variants (MPM) which do not readily evolve in nature.
Novel vector projects under development by Arowor Corp. could circumvent the pharmacoapplied perils associated with other vector technologies such as recombinant AAV and CRISPR. “Our counter-paralysis candidates are engineered with strong vectors having decisive single-gene delivery surveillance complexes.” – Aron Workman, CEO
Aron Workman serendipitously invented superoxide dismutase analogs (SODA) with increased bioactivity at the University of Florida in 2010. Like the highly successful insulin analogs, the superoxide dismutase analogs have engineered advantageous molecular properties which will translate to advantageous pharmaceutical effect.
The Arowor Corp. launch video shows its initial technology and ongoing pipeline development. “A wealth of superoxide dismutase expertise collected over the past 15 years uniquely prepare us for an ever stronger candidacy with broader mass market appeal, which will culminate in the initial launch of superoxide dismutase analog injectable to the United States drug market.” – Aron Workman, CEO
Arowor Corp. is currently raising initial funds and seeking collaborators and partnerships.
For more information, visit the Arowor Corp. website at https://arowor.com; contact Arowor Corp. by email at <contact@arowor.com>.
Aron Workman, CEO
Arowor Corp.
contact@arowor.com
Visit us on social media:
Twitter
LinkedIn
YouTube
Corporate Launch Video - New Year's Day, 2023 - Arowor Corp.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.