Bioelectric Medicine Market worth $39.51 Billion by 2030 - Exclusive Report by InsightAce Analytic
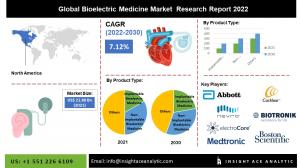
Global Bioelectric Medicine Market is estimated to reach over USD 39.51 billion by 2030, exhibiting a CAGR of 7.12% during the forecast period.
NEW JERSEY, SATTE NJ, USA, October 3, 2022 /EINPresswire.com/ -- The newly published report titled "Global Bioelectric Medicine Market By Product (Implantable Bioelectric Medicine (Cardiac Pacemaker, Implantable Cardioverter Defibrillators (ICD), Spinal Cord Stimulation Device, Cochlear Implants, Deep Brain Stimulation Device, Vagus Nerve Stimulation Device & Others) And Non-Implantable Bioelectric Medicine)- By Trends, Industry Competition Analysis, Covid-19 Analysis, Revenue (US$ Billions) and Forecast Till 2030." of InsightAce Analytic Pvt. Ltd. features detailed industry analysis and an extensive study on the market, exploring its significant factors.
Request Sample: https://www.insightaceanalytic.com/request-sample/1379
The global bioelectric medicine market is estimated to reach over USD 39.51 billion by 2030, exhibiting a CAGR of 7.12% during the forecast period.
Bioengineering, neuroscience, and molecular medicine are all included in bioelectric medicine to develop nerve stimulation technologies for biological parameters during treatment. By using electrical impulses, it alters how the body functions. These medications serve as an alternative to drug-based therapy. The foundation of bioelectric medicine is neurostimulation, the electrical impulse modulation of the neurological system. Major market drivers include the rising prevalence of chronic and acute illnesses, the rising geriatric population, product improvements, and activities by important firms and organizations. By combining bioengineering, molecular medicine, and neurology, bioelectric therapy creates nerve-stimulating devices to control biological processes during disease treatment. The main factor projected to drive the market is the increased prevalence of chronic diseases like cardiac and neurological problems. Other significant drivers of the market's expansion are manufacturers' increasing R&D expenditures and the rate at which regulatory agencies approve new bioelectric medications.
List of Prominent Players in the Bioelectric Medicine Market:
Abbott Laboratories
Boston Scientific Corporation
BIOTRONIK SE & Co. KG
Cochlear Limited
electroCore, Inc.
Inspire Medical Systems, Inc.
LivaNova Plc
Medtronic Plc
MED-EL
Nevro Corp.
Nuvectra Corporation
NeuroMetrix, Inc
OMRON CORPORATION
PIXIUM VISION
Second Sight Medical Products, Inc.
SetPoint Medical
Stimwave LLC
Key Developments: https://www.insightaceanalytic.com/report/global-bioelectric-medicine-market/1379
Market Dynamics:
Drivers-
The older population is more susceptible to neurologic or cardiovascular issues. Due to their higher risk of contracting various diseases, geriatric patients comprise a sizeable fraction of the total patient population. The growth of this population group will ensure a long-term and rising need for bioelectric devices, such as cardiac pacemakers, implanted cardioverter defibrillators, deep brain stimulators, and spinal cord stimulators, to treat cardiovascular and neurological conditions. Significant development opportunities exist for major market players in developing nations, including Malaysia, South Korea, Vietnam, Israel, Africa, the United Arab Emirates, and Saudi Arabia. This is due to their lax regulatory requirements, improved healthcare facilities, expanding patient populations, and rising healthcare costs. This has led important companies in the bioelectric medicine market to concentrate on rising nations along with the escalating competition in mature markets.
Challenges:
One of the main factors limiting market growth is the high cost of bioelectric devices like cochlear implants and neurostimulators, especially in price-sensitive countries like Latin America, Asia, and Africa. In emerging nations, particularly in Mexico and Brazil, healthcare providers typically lack the financial means to invest in advanced technologies. Additionally, the crew needs to receive intensive training in the proper use and upkeep of bioelectric devices. Neurosurgeons who specialize in neural implant procedures and have completed a functional neurosurgery fellowship are required to guide the implantation of bioelectric devices such as neurostimulators in the appropriate body part. There is now a considerable bioelectric shortage of neurologists available worldwide. Given the anticipated rise in healthcare demand due to variables, including the expanding patient population and increasing illness prevalence, this shortfall will be of concern.
Regional Trends:
In the forecast period, North America dominates the market due to medical device producers like Abbott and Boston Scientific Corporation. With the rising need for cutting-edge medical gadgets for treatment, North America is a leading regional market. Additionally, the region's highly advanced healthcare system and accessibility to cutting-edge items have driven the local market expansion. Large bioelectric medicine producers and highly developed healthcare infrastructure are anticipated to continue to be key market drivers. During the predicted period, the Asia Pacific region is anticipated to increase because of the growing elderly population in Asian nations like China and India. Additionally, chronic disorders such as cardiac arrhythmias, Alzheimer's disease, Parkinson's disease, and epilepsy are becoming more common in this area, which is anticipated to increase the use of bioelectric.
Recent Developments:
• In March 2021, Medtronic plc, a global leader in medical technology, announced that the food and drug administration (FDA) approved modified commercial labeling for the IntellisTM Platform with Differential Target Multiplexed (DTMTM) programming for the chronic disease treatment of persistent back and leg pain. The updated labeling will incorporate study results from a multicenter randomized control trial showing that differential target multiplexed spinal cord stimulation (DTM SCS) provides improved back pain alleviation compared to conventional spinal cord stimulation (SCS).
• In January 2021, Boston Scientific Corporation gained food and drug administration (FDA) approval for its fourth-generation Vercise GenusTM Deep Brain Stimulation (DBS) System. The portfolio, which has been cleared for conditional use in an MRI environment, consists of a variety of Bluetooth-enabled, rechargeable and non-rechargeable implantable pulse generators (IPGs) that power CartesiaTM Directional Leads, which are designed to deliver optimal symptom alleviation.
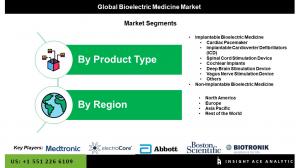
Segmentation of Bioelectric Medicine Market-
By Product
• Implantable Bioelectric Medicine
o Cardiac Pacemaker
o Implantable Cardioverter Defibrillators (ICD)
o Spinal Cord Stimulation Device
o Cochlear Implants
o Deep Brain Stimulation Device
o Vagus Nerve Stimulation Device
o Others
• Non-Implantable Bioelectric Medicine
By Region-
• North America-
o US
o Canada
o Mexico
• Europe-
o Germany
o UK
o France
o Italy
o Spain
o Rest of Europe
• Asia-Pacific-
o China
o Japan
o India
o South Korea
o South East Asia
o Rest of Asia Pacific
• Latin America-
o Brazil
o Argentina
o Rest of Latin America
• Middle East & Africa-
o GCC Countries
o South Africa
o Rest of the Middle East and Africa
• Others
For Information: https://www.insightaceanalytic.com/report/global-bioelectric-medicine-market/1379
Priyanka Tilekar
Insightace Analytic Pvt. Ltd.
551-226-6109
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.