PT150 FOR TREATMENT OF “LONG HAUL COVID” FEATURED POSTER AT THE NEUROSCIENCE CLINICAL-TRANSLATIONAL RESEARCH SYMPOSIUM
Palisades Therapeutics’ PT150 for symptoms associated with long haul COVID syndrome requested for possible inclusion in the NIH RECOVER COVID Studies
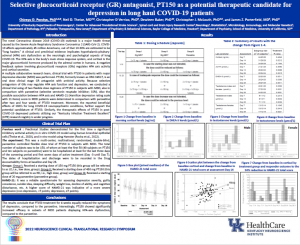
A multi-center, randomized, double-blind, paroxetine-controlled, flexible dose study with PT150 in patients with major depressive disorder was conducted. The Hamilton Rating Scale for Depression (HDRS), the most widely used clinician-administered depression assessment scale, was administered to determine clinical efficacy. PT150 treatment for 4-weeks equally reduced the symptoms of depression measured by HDRS score compared to the selective serotonin reuptake inhibitor (SSRI) paroxetine. In particular, PT150 showed significantly increased efficacy in subsets of depressed patients displaying HPA-axis dysfunction (as measured by high levels of serum cortisol), compared to paroxetine. Similarly, in another clinical trial, we also observed that the treatment of PT-150 is particularly effective in depressed patients with HPA-axis dysregulation compared to the antidepressant clomipramine. Importantly, our collaborators have also demonstrated for the first time a significant inhibitory antiviral activity in in vitro COVID-19 model using human bronchial epithelial lining cells (Theise et al., 2020), and in vivo COVID-19 infection model using Syrian hamster (Rocha et al., 2022). These data have been accepted for presentation at the 2022 Neuroscience Clinical-Translational Research Symposium at the University of Kentucky.
Since, neuroinflammatory changes have been implicated in long haul COVID syndrome, underlying symptoms such as depression, fatigue, memory dysfunction, and “brain fog”, it is postulated that cortisol-mediated neuroinflammation can be down regulated by PT150. Pre-clinical data for PT150 strongly supports these findings, with protective effects against models of viral/infectious neuroinflammation (lipopolysaccharide, tumor necrosis factor, poly I:C), decreased pro-inflammatory interleukins (IL-6 and IL-1B), and inhibition of SARS-CoV-2 microgliosis in brains of infected Syrian hamsters. RNA silencing of glucocorticoid receptor in these models mitigates the benefits of PT150 further supporting the HPA-axis modulatory effects as a primary and targeted therapeutic against neuroinflammation underlying long haul COVID-19. Palisades’ lead scientist Dr. Neil Theise states “Not only do we expect beneficial results for symptoms of long haul COVID, but the direct antiviral actions of PT150 are likely to diminish or eliminate potential rebound infections as have been seen following other anti-covid treatments.” Moreover, in support of Palisades Therapeutics, University of Kentucky’s faculty and Palisades’ scientific advisor, Dr. Chirayu D. Pandya and his collaborators are planning to evaluate the therapeutic effects of PT150 in post-COVID-19 depressed patients utilizing “Kentucky Infection Treatment Excellent” (KITE) research registry, University of Kentucky.
In light of the potential of PT150 for therapeutic benefit in patients with long haul COVID neurological and psychological symptoms, PT150 has been requested for possible inclusion in the NIH RECOVER COVID Clinical Studies.
Palisades invites leading companies such as Glaxo Smith Kline (NYSE: GSK), Johnson & Johnson (NYSE: JNJ) and Pfizer Inc. (NYSE: PFE) to review our data.
Randi Altschul
Pop Test Oncology/Palisades Therapeutics
+1 201-943-3770
randi@palisadestherapeutics.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.