Nikkei BP releases The Directory of Biotech Startups in Japan 2021-2022
Nikkei BPs new The Directory of Biotech Startups in Japan 2021-2022 was published to help bring together Japanese biotech startups and global investors.
TOKYO, JAPAN, September 21, 2022 /EINPresswire.com/ -- In August 2022, Nikkei Business Publications published an English edition of The Directory of Biotech Startups in Japan 2021-2022. This directory was originally released in Japanese in June 2021, and it was compiled by the editorial team of Nikkei Biotechnology & Business. The aim of the work is to encourage active collaboration between Japan’s biotech startups and global investors. The text was translated from Japanese to English using the Machine Translation Assistance System, developed by Nikkei, Inc. This new edition runs 1046 pages and is available in PDF format; it is priced at USD9,950 per copy, not including tax.Nikkei Biotechnology & Business is a specialized biotechnology publication with over 40 years of history in Japan. In cooperation with major universities and prominent venture capitalists throughout Japan, it regularly surveys startups in the biotechnology field. From among them, it has selected 395 with outstanding technologies and business models, which are highlighted in The Directory of Biotech Startups in Japan 2021-2022.
Despite their technological prowess, Japan’s biotech startups face challenges when it comes to disseminating information overseas. This is because many do not have their own websites, and they tend to disclose less information than larger, listed companies. To address this issue, the editors of Nikkei Biotechnology & Business contacted all of the startups in the directory to research their technologies, patents, collaborators, and shareholders, as well as to gather other types of information.
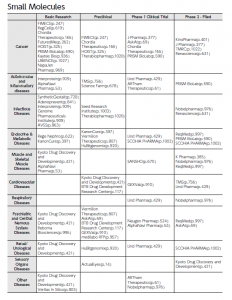
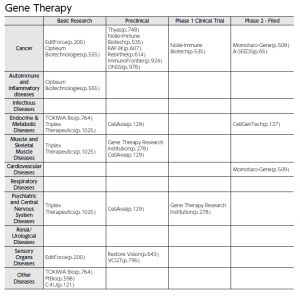
Additionally, the editors conducted interviews with 207 companies of particular note to analyze their technological features and management strategies. The resulting detailed company reports are available in a unified format for easier comparison by pharmaceutical giants and venture funds around the world looking for new business partners. Industry maps produced based on modality are included in order to facilitate screening procedures. Products developed by the startups are categorized into 11 tables focused on the following modalities: “Small molecules,” “Peptides,” “Antibodies (including antibody-drug conjugates (ADCs)),” “Proteins,” “Nucleic Acids,” “Gene Therapy,” “Cell Medicine,” “Regenerative Medicine,” “Vaccines (including Adjuvants),” “Other modalities” and “Digital (Digital Health and Digital Therapeutics).” For each modality, products are plotted on a matrix (see figures), with disease areas presented vertically and relevant development stages horizontally. Using this type of visualization, startups of interest can be easily identified based on modality, disease area, and development stage.
【Fig1】
【Fig2】
The Directory of Biotech Startups in Japan 2021-2022 is sold by Research and Markets, the world’s largest-class market research store, and though other means. Research and Markets makes reports available for over 800 industries, from the automotive business to telecommunications and zoology, and it has more than 450 companies on the Fortune 500 list as clients.
https://www.researchandmarkets.com/r/tlf6an
For further details, please contact:
Nikkei Business Publications, Inc.
( https://nkbp.jp/inquiry-e )
Creating Work: My Resume (Revised and Updated Edition)
Exploring the Future Five Years from Now
Top Five Keywords for Foreseeing the Healthcare Industry in 2022
Public Relations Office
Nikkei Inc.
pr@nex.nikkei.co.jp
Visit us on social media:
Facebook
Twitter
LinkedIn
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.