The continuous and semi-continuous bioprocessing market by Roots Analysis
Roots Analysis has announced the addition of “Continuous and Semi-Continuous Bioprocessing Market, 2021 – 2030” report to its list of offerings.
LONDON, ENGLAND, UNITED KINGDOM, September 8, 2021 /EINPresswire.com/ -- The surge in demand for COVID-19 vaccines has presented lucrative opportunities for both innovators and contract service providers having continuous and semi-continuous manufacturing capabilities for biointensification
Roots Analysis has announced the addition of “Continuous and Semi-Continuous Bioprocessing Market, 2021 – 2030” report to its list of offerings.
Given the growing pipeline of biological drugs, and the rising preference for such therapeutic interventions, the demand for cost-effective biomanufacturing processes has increased. As a result, several innovators and contract service providers are evaluating the potential of continuous and semi-continuous upstream and downstream bioprocessing technologies, owing to their various advantages.
To order this 190+ page report, which features 60+ figures and 115+ tables, please visit this https://www.rootsanalysis.com/reports/continuous-and-semi-continuous-bioprocessing-market.html
Key Market Insights
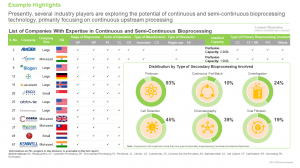
Presently, more than 70 companies claim to have capabilities for continuous manufacturing of biologics
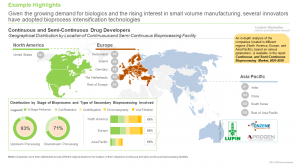
Close to 45% of the continuous and semi-continuous bioprocessing companies are headquartered in Europe; further, around 20% of these companies have established their dedicated facilities in other geographical regions, such as North America and Asia-Pacific.
Over 65% of the installed continuous upstream manufacturing capacity belongs to established players
More than 40% of the global continuous upstream processing capacity is installed in Europe, followed by Asia-Pacific (33%). Further, close to 55% of the capacity belongs to the facilities owned by contract service providers.
Continuous and semi-continuous bio-intensification approach has potential to save ~40% of the overall biopharmaceutical manufacturing cost
By 2030, we expect that adoption of continuous and semi-continuous approach is likely to enable the net, annual cost savings of close to USD 50 billion.
By 2030, the opportunity within the continuous and semi-continuous bioprocessing market is likely to be over USD 500 million
Presently, the use of continuous and semi-continuous bio-intensification approach is largely restricted to the developed nations, and the majority of revenues from biologics manufactured via this approach are distributed between North America (~25%) and Europe (~40%). Once this process is adopted for the end-to-end manufacturing of biologics, estimates in the report suggest are likely to grow at even higher pace.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/continuous-and-semi-continuous-bioprocessing-market.html
Key Questions Answered
Who are the leading contract manufacturers with expertise in continuous and semi-continuous bioprocessing?
Who are the leading innovators / drug developers with expertise in continuous and semi-continuous bioprocessing?
In which regions are majority of the continuous and semi-continuous bioprocessing facilities located?
What is the likely cost saving potential of continuous and semi-continuous bioprocessing technology?
What is the currently installed global capacity for continuous and semi-continuous bioprocessing?
How is the current and future market opportunity likely to be distributed across key market segments?
The USD 500+ million (by 2030) financial opportunity associated with continuous and semi-continuous bioprocessing services market has been analyzed across the following segments:
Type of Manufacturer
Innovator / Drug Developer
Contract Service Provider
Company Size
Large
Mid-sized
Small
Scale of Operation
Preclinical / Clinical
Commercial
Stage of Bioprocess
Upstream Bioprocessing
Downstream Bioprocessing
Geographical Regions
North America
Europe
Asia-Pacific
MENA
Latin America and Rest of the World
The report also features inputs from eminent industry stakeholders, according to whom, continuous and semi-continuous bioprocessing technologies are likely to witness a significant increase in the adoption rate in the near future, given their advantages over the traditional bioprocessing technologies. The report includes detailed transcripts of discussions held with the following experts:
Jon Coffman (Senior Director of Bioprocess Technology and Engineering, AstraZeneca)
Ehsan Mahdinia (Assistant Professor, Albany College of Pharmacy and Health Sciences)
Himanshu Gadgil (Director and Chief Scientific Officer, Enzene Biosciences)
The research includes profiles of key players (listed below), featuring a brief overview of the company, financial information (if available), details on its service portfolio, continuous and semi-continuous bioprocessing capabilities, scale of operation, stage of bioprocess, types of biologics manufactured, continuous and semi-continuous bioprocessing manufacturing facilities, recent developments and an informed future outlook.
AGC Biologics
Biogen
Bristol-Myers Squibb
Enzene Biosciences
FUJIFLM Diosynth Biotechnologies
Merck KGaA
Novasep
Sanofi Genzyme
UCB Pharma
WuXi Biologics
For additional details, please visit
https://www.rootsanalysis.com/reports/continuous-and-semi-continuous-bioprocessing-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Pharmaceutical Contract Research Services Market: Industry Trends and Global Forecasts, 2021-2030
2. Lipid Contract Manufacturing Market: Industry Trends and Global Forecasts, 2021-2030
3. Biologics Fill / Finish Services Market (2nd Edition): Industry Trends and Global Forecasts, 2021-2030
4. Vaccine Contract Manufacturing Market (3rd Edition): Industry Trends and Global Forecasts, 2021-2030
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
ben.johnson@rootsanalysis.com
Gaurav Chaudhary
Roots Analysis
+1 415-800-3415
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.