Kent Imaging Inc. Receives FDA 510(k) Clearance on SnapshotNIR V3.0 (KD204)
Advanced near infrared spectroscopy imaging for instant and nonivasive tissue oxygen assessment
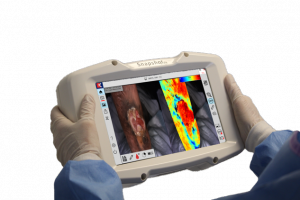
CALGARY, AB, CANADA, January 6, 2021 /EINPresswire.com/ -- Kent Imaging announced today the FDA 510(k) clearance for SnapshotNIR V3.0 (KD204) – now available for shipping. This upgrade provides significant feature enhancements to the imaging technology that advances tissue assessment in acute and chronic wounds. The multitude of advancements include the ability to image most skin tones (overcoming the melanin barrier), linear and surface area wound measurements, easy report generation with image comparisons, and enhanced patient file management, to name a few.
SnapshotNIR utilizes near infrared light to determine tissue oxygen saturation (StO2), which is a key indicator of tissue health. Ideal for microcirculation assessment, it conveys a comprehensive picture of the healing capacity of wounds or surgical tissue. This critical information is used to support clinical judgement in choosing, evaluating, and tracking treatment and surgical options throughout the care continuum.
“We are excited to release this enhanced version of SnapshotNIR as part of Kent’s dedication to bringing innovation to this critical market. Robust features asked for by front-line wound care specialists and reconstructive surgeons have been incorporated, delivering a product that can easily track and document wound healing progress and support therapeutic decisions,” noted Pierre Lemire, CEO.
About Kent Imaging Inc.
Kent Imaging, located in Calgary, Alberta, Canada, is a leading innovator in oxygenation imaging, who develops, manufactures, and markets medical technology that supports real-time decision making in wound care, vascular and surgical sub-specialties. Kent holds multiple patents in oxygen imaging technology and continues to provide innovative and advanced diagnostic imaging solutions to aid healthcare systems nationally and internationally. For more information about Kent Imaging, visit www.kentimaging.com.
Sandra Jenks
Kent Imaging Inc.
+1 403-455-7610
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.