InVivo Analytics Awarded $1.7 million from National Cancer Institute for Next Generation Fluorescence Tomography
Follow-on SBIR Phase II award for development of preclinical 3D fluorescence imaging and automated biodistribution analysis of spatial T-cell migration
Using fluorescence optical imaging technologies for biodistribution studies will accelerate immuno-oncology therapeutic development.”
NY, USA, December 15, 2020 /EINPresswire.com/ -- Announcement: The National Cancer Institute (NCI) of the National Institutes of Health (NIH) awards InVivo Analytics® Inc. $1.7 million in Small Business Innovation Research (SBIR) Phase II funding to develop and commercialize an automated 3D fluorescence imaging and biodistribution analysis platform, InVivoFLUOR™, for preclinical imaging studies of immuno-oncology mouse models. Small animal research is the cornerstone of preclinical research, with small animal imaging studies playing a pivotal role in moving new cancer immunotherapies from the bench to the bedside.— Dr. Alexander Klose, co-founder and CTO
The Company: InVivo Analytics is an early stage technology company focused on cross-platform hardware and software solutions to automate preclinical imaging and data analysis. InVivoAX, the company's cloud-based image processing and data analysis platform, centralizes mouse image and data visualization with a browser-based interface. This is accomplished with novel hardware units that connect the existing base of preclinical imaging systems to the InVivoAX cloud software. For optical imaging, InVivoFLUOR constitutes a hardware plug-in for optical imaging systems that enables fluorescence tomography in addition to the company’s recently commercialized InVivoPLOT unit for bioluminescence tomographic imaging.
What is an SBIR?: InVivoFLUOR is a high-risk, high-reward technology, thus InVivo Analytics sought SBIR programmatic funding from the NIH/NCI to support its development efforts. The SBIR mechanism is a highly competitive program that gives domestic small businesses access to the Federal Research/Research and Development (R/R&D) budget with the potential for commercialization. The SBIR program funds a diverse portfolio of startups and early stage companies across technology areas and markets to stimulate technological innovation and increase commercialization by transitioning R&D into high impact technologies.
“The mission of the SBIR/STTR programs is to support scientific excellence and technological innovation through the investment of Federal research funds in critical American priorities to build a strong national economy.” (SBIR.gov).
This SBIR Phase II was awarded after InVivo Analytics successfully demonstrated technological feasibility during the SBIR Phase I ($222k) in 2019. In Phase II, InVivo Analytics will develop InVivoFLUOR for fluorescence tomography and automated biodistribution analysis of mouse models using the preclinical testing of ovarian cancer immunotherapeutics as a model system.
The Problem: Immunotherapy (IMT) is a cancer treatment that harnesses activated T-cells to induce a targeted immune response against the cancer. Preclinical IMT research has been limited because there are no accessible methods to longitudinally follow T-cell migration in live mouse cancer models. This is an urgent unmet need because the only viable methods to monitor tumor infiltrating T-cells, immunohistochemistry and cell sorting, are both terminal, ex vivo and variable.
Fluorescence imaging (FLI) is an essential technology in the preclinical space for tracking targets such as drugs and cancers, but quantifying these labeled targets in vivo has been relegated to semi-quantitative technologies at best. FLI offers many advantages including the high-throughput, ease of use, and low cost; however, fluorescence light is dependent on the optical tissue type, and on the animal’s size, shape, and pose. Furthermore, direct FLI of animals does not provide the in vivo organ load of T-cells, also termed biodistribution, because three-dimensional (3D) spatial maps of fluorescence T-cells cannot be determined from only two-dimensional (2D) images.
Additionally, there is no compatible anatomical atlas for organ delineation, thus manual region-of-interest (ROI) or organ delineation is the norm. Manual image data analysis is highly operator-dependent and time-consuming, resulting in low reproducibility and high variability. Automating image analysis has not been feasible using current imaging techniques because of the absence of a rigid spatial framework tracking data points.
Solution: InVivo Analytics has addressed the urgent unmet need for obtaining the in vivo biodistribution of T-cells by developing InVivoFLUOR. InVivoFLUOR enables 3D mapping and visualization of fluorescent-labeled T-cells inside a living mouse. The InVivo Analytics team also calculated in vivo T-cell biodistribution by automatically aligning the 3D fluorescence maps to an anatomical organ atlas. This operator-independent organ delineation process and spatial alignment is a major leap towards automating in vivo biodistribution analysis and, thus, enables developing new IMTs by providing go/no-go quantitative information whether the therapeutic T-cells are delivered to the target cancer cells.
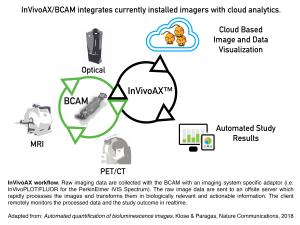
The key technology behind InVivoFLUOR’s fluorescence biodistribution analysis is a hardware plugin module for optical imaging systems and a fast fluorescence tomography algorithm running in the cloud. InVivo Analytics leverages the company's patented Body-Conforming Animal Mold (BCAM), which places the animal in a known position and defined geometry, which subsequently allows for fast fluorescence image reconstruction and coregistration to a mouse atlas. The image data is uploaded to the company’s cloud-based image analysis platform InVivoAX.
InVivoFLUOR will be validated using an immuno-oncology (IO) preclinical mouse model testing the efficacy of an advanced immunotherapy (IMT) from the University of Washington. This IMT harnesses activated T-cells to induce a targeted immune response against ovarian cancer. Preclinical IMT research has been hampered by the inability to longitudinally determine T-cell biodistributions in mouse models. InVivoFLUOR will calculate fluorescence-labeled T-cell spatial distribution and co-register them to 3D maps of disseminated ovarian tumors in the live mouse IMT model.
InVivoAX Unifies Optical and Nuclear Imaging: InVivoFLUOR will be added to the company’s InVivoAX platform, a cloud-based Software-as-a-Service (SaaS) for the pharmaceutical industry and research institutions. This platform comprises different modules as add-ons to the installed base of preclinical imaging systems, including bioluminescence imaging and Positron Emission Tomography (PET) systems. Because it combines different imaging modalities within a network of compatible BCAMs, it will enable cross-platform data comparison and analysis, eliminate operator-dependent variability, increase data reproducibility, and will facilitate the translation of new therapeutics.
“The InVivoAX automated software addresses the need for reproducible and unbiased data analysis by removing the operator’s cognitive bias from determining regions of interest.” Explains co-founder and CEO Dr. Neal Paragas.
As part of the paradigm changing InVivoAX suite of preclinical image analysis modules, InVivoFLUOR will provide a new way of preclinical image analysis to fields such as immuno-oncology and gene therapy. Importantly, the InVivoFLUOR platform is maturing at an opportune moment where there is an urgent unmet need for new research tools for non-destructive biodistribution studies.
Neal Paragas
InVivo Analytics, Inc.
+1 917-499-5951
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.