TCR Engineered T-Cell Therapy Status Report A Must Have Study for Academia and Pharma/Biotech Companies
The new report covers global companies like Adaptimmune, Bellicum Pharmaceuticals, Cell Medica, GlaxoSmithKline and 30+ companies.
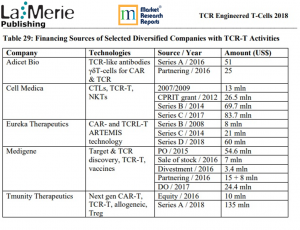
Cell Medica has entered into an exclusive license and option agreement with UCL Business, the technology commercialisation company of UCL, for the dominant TCR platform patent and two target antigens.”
LEWES, DELAWARE, DELAWARE, UNITED STATES, August 21, 2018 /EINPresswire.com/ -- T-Cell Receptors (TCR) and Chimeric Antigen Receptors (CAR) are the cutting edge of adoptive T-cell therapy. Both receptors deploy T-cells to target the tumor, but CAR T-cells (CAR-T) are limited to binding to cell surface antigens, while TCR T-cells (TCR-T) recognize peptides (derived from intracellular proteins) presented on the cell surface by the major histocompatibility complex (MHC) class I.— LM
Adoptive T-cell therapy has come of age with the recent approvals of two CAR-T therapeutics targeting CD19 (Kymriah and Yescarta) demonstrating feasibility of clinical development with impressive results and of personalized manufacturing & logistics. It remains to be seen whether these autologous CAR-Ts will live up commercial expectations, as first quarter 2018 sales of Kymriah were far below expectations (only US$ 12 mln). The final prices of US$ 475,000 (Kymriah) and US$ 373,000 mln (Yescarta) are challenging the commercial viability of autologous CAR-T therapy. Allogeneic cell therapy might be one technological solution for the pricing problem.
While CAR-Ts performed very well for hematologic malignancies, they did less for solid tumors. Furthermore, CAR-T therapy is restricted to cell surface proteins which constitute about 20-25% of all available targets on a solid tumors, leaving nearly 80% of intracellular targets untapped by CAR-T therapy or by conventional antibody therapy.
The concept of TCR T-cell therapy actually predates CAR-Ts, but suffered a series of setbacks due to toxicity caused by cross-reactivity with related and unrelated peptides prompting the development of much more sophisticated specificity screening systems. But TCR-Ts are catching up as evidenced by ten different company-related TCR-Ts entering clinical development in the last two years and further nine in preparation of clinical trials. Significant deals between TCR-T companies and major Pharma/Biotech further underline the promise of TCR-T technologies.
In this new report "TCR Engineered T-Cell Therapy 2018: an industry analysis of technologies, pipelines, stakeholders & deals" brings you up-to-date and gives you answers to key questions about
>target discovery & selection,
>technologies for generation of TCRs,
>neoantigen-specific TCRs,
>off-the-shelf TCR-Ts,
>the TCR-T pipeline,
>manufacturing strategies,
>next generation TCR-Ts,
>key players,
>competition,
>corporate strategy,
>deals and acquisitions and
>financing.
The report can be acquired at MarketResearchReports.com at (https://www.marketresearchreports.com/la-merie-publishing/tcr-engineered-t-cell-therapy-2018-industry-analysis-technologies-pipelines ). There you will find samples pages, a detailed Table of Contents, a list of Tables and of companies discussed in the report.
Browse more reports from our Pharma and Healthcare Category: Therapeutic , Drug Pipeline
Sudeep Chakravarty
Market Research Reports Inc.
+1-302-703-9904
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.