MindRhythm Completes Enrollment in its Multicenter Prehospital Trial for Identifying Large Vessel Occlusion Stroke
CUPERTINO, CALIFORNIA, UNITED STATES, December 4, 2023 /EINPresswire.com/ -- MindRhythm Incorporated, a neurodiagnostic technology company based out of Cupertino, California, today announced that it reached the number of patients required to complete enrollment in its pivotal stroke trial-EPISODE. This trial was designed to validate that MindRhythm’s Harmony can accurately identify LVO stroke prehospital, with the goal of improving triage decisions. Better quality information will help EMS teams ensure a critical patient is transported to a hospital equipped with the necessary treatment resources. Proper triage can be the the difference between life, death, and debilitation.
EPISODE-hEadpulse for Ischemic StrOke DEtection Prehospital, is a large, EMS based study that included 6 states, 17 hospitals, and 14 EMS facilities participating in research resulting in EPISODE being the largest study of a prehospital stroke identification device. Primary Investigator James Paxton, MD from Wayne State Medical in Detroit, Michigan leads the research with the support of 18 subinvestigators throughout the country. The broad participation in the research provides a demographic representation of the diverse population that will benefit from the Harmony stroke identification technology.
Current practice for identifying stroke, in the absence of brain imaging, is based on presentation of symptoms seen during the event. These symptoms are common in many other clinical scenarios as well, confounding stroke assessment, making the subjective process difficult to perform. Adding a technology that can objectively determine the presence of large vessel occlusion strokes has the potential to significantly improve immediate detection and direct transport to the appropriate facility. This benefit, is the purpose of the EPISODE study.
EPISODE was composed of two parts. The first, was designed to test the hypothesis that the device can identify LVO stroke with a sensitivity and specificity noninferior to a commonly used stroke scale: LAMS (Los Angeles Motor Scale). Part 1 also focused on collecting user feedback from the field to iterate the physical design of the device to match user needs for integration into standard workflows reducing barriers to adoption. The released results of phase 1 significantly proved the initial hypothesis. A blinded part 2 was designed to replicate the results of part 1 while powered for superiority to stroke scales in preparation for FDA clearance. The data for part 2 is currently being unblinded and analyzed, but there is confidence that its goals will be met. While any stroke can be debilitating, LVO is the most debilitating and often fatal type of stroke. MindRhythm’s technology is designed to improve outcomes for patients by ensuring that at the start of care, the best treatment decision for the patient is made.
MindRhythm was granted a Breakthrough Device Designation by the FDA as a result of the part 1 data from EPISODE and will use the combined data sets from part 1 and 2 to submit for clearance with commercialization to follow.
About the Harmony Headset
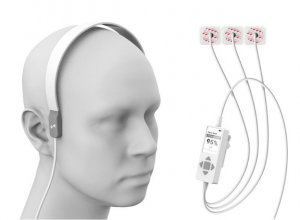
Harmony is a novel, noninvasive medical device designed to aid in the rapid identification of Large Vessel Occlusion (LVO) strokes, the most debilitating of all stroke types. Harmony monitors a newly discovered physiology MindRhythm calls the “HeadPulse”. The HeadPulse is measured by applying a highly sophisticated sensor to the patient’s head, measuring minute pulsations produced by each heartbeat. The HeadPulse changes dramatically during LVO strokes, which is read by Harmony. Coupling the Harmony results with a simple clinical examination of stroke, the device can discriminate LVO strokes among stroke patients in a matter of seconds.
About MindRhythm
MindRhythm is a medical technology company focused on preventing neurological injury. Founded by world-renowned scientific experts with significant commercialization success, MindRhythm’s monitoring technologies provide real-time visibility to life-threatening situations at home, prehospital, in the operating room, and on the field. MindRhythm’s technologies allow clinicians to intervene, optimize and manage care to prevent brain damage. Collaborating with the healthcare community, MindRhythm looks to apply a systematic approach to reducing time to treatment in strokes and monitor neurological health during recovery from injury. Together, let’s save lives and improve the quality of life: https://mindrhythm.com
EPISODE-hEadpulse for Ischemic StrOke DEtection Prehospital, is a large, EMS based study that included 6 states, 17 hospitals, and 14 EMS facilities participating in research resulting in EPISODE being the largest study of a prehospital stroke identification device. Primary Investigator James Paxton, MD from Wayne State Medical in Detroit, Michigan leads the research with the support of 18 subinvestigators throughout the country. The broad participation in the research provides a demographic representation of the diverse population that will benefit from the Harmony stroke identification technology.
Current practice for identifying stroke, in the absence of brain imaging, is based on presentation of symptoms seen during the event. These symptoms are common in many other clinical scenarios as well, confounding stroke assessment, making the subjective process difficult to perform. Adding a technology that can objectively determine the presence of large vessel occlusion strokes has the potential to significantly improve immediate detection and direct transport to the appropriate facility. This benefit, is the purpose of the EPISODE study.
EPISODE was composed of two parts. The first, was designed to test the hypothesis that the device can identify LVO stroke with a sensitivity and specificity noninferior to a commonly used stroke scale: LAMS (Los Angeles Motor Scale). Part 1 also focused on collecting user feedback from the field to iterate the physical design of the device to match user needs for integration into standard workflows reducing barriers to adoption. The released results of phase 1 significantly proved the initial hypothesis. A blinded part 2 was designed to replicate the results of part 1 while powered for superiority to stroke scales in preparation for FDA clearance. The data for part 2 is currently being unblinded and analyzed, but there is confidence that its goals will be met. While any stroke can be debilitating, LVO is the most debilitating and often fatal type of stroke. MindRhythm’s technology is designed to improve outcomes for patients by ensuring that at the start of care, the best treatment decision for the patient is made.
MindRhythm was granted a Breakthrough Device Designation by the FDA as a result of the part 1 data from EPISODE and will use the combined data sets from part 1 and 2 to submit for clearance with commercialization to follow.
About the Harmony Headset
Harmony is a novel, noninvasive medical device designed to aid in the rapid identification of Large Vessel Occlusion (LVO) strokes, the most debilitating of all stroke types. Harmony monitors a newly discovered physiology MindRhythm calls the “HeadPulse”. The HeadPulse is measured by applying a highly sophisticated sensor to the patient’s head, measuring minute pulsations produced by each heartbeat. The HeadPulse changes dramatically during LVO strokes, which is read by Harmony. Coupling the Harmony results with a simple clinical examination of stroke, the device can discriminate LVO strokes among stroke patients in a matter of seconds.
About MindRhythm
MindRhythm is a medical technology company focused on preventing neurological injury. Founded by world-renowned scientific experts with significant commercialization success, MindRhythm’s monitoring technologies provide real-time visibility to life-threatening situations at home, prehospital, in the operating room, and on the field. MindRhythm’s technologies allow clinicians to intervene, optimize and manage care to prevent brain damage. Collaborating with the healthcare community, MindRhythm looks to apply a systematic approach to reducing time to treatment in strokes and monitor neurological health during recovery from injury. Together, let’s save lives and improve the quality of life: https://mindrhythm.com
Beth Johnson
MindRhythm Incorporated
+1 860-836-8630
Beth.Johnson@MindRhythm.com
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.