NDR Medical Technology’s ANT-X Granted FDA Clearance, Paving the Way for Image-guided Surgeries
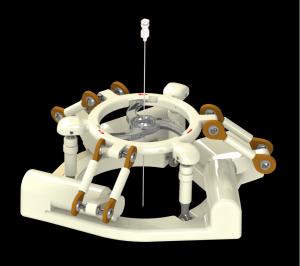
ANT-X is an interventional robot that leverages C-arm fluoroscopy to help clinicians achieve swift and accurate percutaneous needle placement.
SINGAPORE, July 10, 2023/EINPresswire.com/ -- NDR Medical Technology, an AI-empowered interventional robotics company, today announced that the ANT-X has been granted FDA 510(k) clearance by the U.S. Food and Drug Administration (FDA). This clearance makes ANT-X the world’s first automated robotic device to aid in needle positioning and alignment to access the kidney for Percutaneous Nephrolithotomy (PCNL), a urology procedure for kidney stone removal. It is designed to empower clinicians to perform image-guided Fluoroscopic percutaneous access with speed and precision.Approximately 10 percent of the American population is affected by kidney stones in their lifetime1, and there is a pressing need for effective treatment. Among treatment options available for patients, PCNL stands out as the preferred treatment of choice for patients2, as it tends to see higher stone-free rates compared with procedures such as ureteroscopy3. Despite its benefits, PCNL represents only an estimated 7-8 percent of stone procedures conducted in the US today 4. With FDA approval secured for the ANT-X, clinicians will be able to carry out PCNL procedures safely and effectively for a larger number of patients
“As urologists, we recognise that achieving percutaneous access to the renal collecting system is a crucial step that significantly impacts the success of kidney stone surgery,” said Dr Kazumi Taguchi, Assistant Professor and head of research of Nephro-urology at Nagoya City University, “Despite advancements in surgical techniques, we are still faced with the daunting task of performing this procedure, which demands considerable time and effort.”
With the advancement of PCNL techniques such as mini PCNL and ultra-mini PCNL, more patients can be discharged on the same day without the need for stents, tubing, or any remaining stones5.
"Recent trends show an increase in the use of PCNL targeting stones up to 2cm, while Mini PCNL has been proven to minimise adverse events. We are confident that the adoption of ANT-X reduces the complexity of such procedures.” said Alan Goh, CEO, NDR Medical Technology.
In the US, current reimbursement codes cover procedures for stone sizes up to 2cm or larger, with new updates to the codes allowing urologists to be incentivised for performing their own access in PCNL procedures6. With ANT-X, urologists can now confidently perform image-guided access, which was previously challenging without assistance.
NDR Medical Technology also recently placed second in the MedTech category at SelectUSA Tech 2021, a showcase for entrepreneurs at the SelectUSA Investment Summit 2021, organised by the US Department of Commerce.
Beyond urology procedures, the versatility of ANT-X extends to other indications. In Singapore, the company successfully conducted its first Neurospine procedure, discoplasty, in June 2023. Driven by a vision to revolutionise the standard of care, NDR Medical Technology is seeking partners and investors to join in the mission of creating breakthrough solutions that transform the future of healthcare.
“Achieving FDA 510(k) approval gives NDR Medical Technology access to the largest healthcare market in the world, and is a critical step ahead for the company,” said Hsien-Hui Tong, Executive Director – Investments, SGInnovate. “This milestone is a sign of consistent good traction made by the team as it continues to expand the applications of its technology, and deliver value to its customers, partners and investors.”
###
For media and other queries, please contact:
Evangeline Chia
evangeline@ndrmedical.com
About NDR Medical Technology Pte Ltd
NDR Medical Technology was founded in 2015 and developed the patented Automated Needle Targeting system (ANT) that can facilitate safe and accurate needle punctures to organs such as the lungs, kidney, pancreas, and spine. Integrating AI and robotics, we empower surgeons to conduct pioneer image-guided robotic procedures with improved accuracy, precision, and safety for patients.
Our first interventional robot (ANT-X) integrates C-arm fluoroscopy and AI software to assist clinicians in percutaneous needle placements and can be used in a wide range of procedures such as urology, neurology, and orthopedics. ANT-C is a smart lesion targeting system that AI uses CT-can images to automate lesion detection, needle path planning and needle targeting with unparalleled precision and accuracy. Applications include usages in pulmonology and hepatology. As a cut above the rest, our robots cost less than half of the current technology out in the market and can function without the need for external sensors.
With a renewed vision to lead AI-empowered interventional robotics, we strive to create revolutionary breakthroughs in the industry and transform the healthcare landscape.
References:
1 - https://www.orlandohealth.com/content-hub/why-southerners-have-a-higher-risk-of-kidney-stones
2 - https://www.sciencedirect.com/science/article/pii/S1743919116310378
3 - https://www.urologytimes.com/view/mini-pcnl-has-higher-stone-free-rate-than-ureteroscopy-and-similar-cost-burden
4 - Chung KJ, Kim JH, Min GE, Park HK, Li S, Del Giudice F, Han DH, Chung BI. Changing Trends in the Treatment of Nephrolithiasis in the Real World. J Endourol. 2019 Mar;33(3):248-253. doi: 10.1089/end.2018.0667. Epub 2019 Feb 13. PMID: 30628473.
5 - https://reports.mountsinai.org/article/uro2022-11-ultra-mini-pcnl-a-new-approach-to-treating-kidney-stones
6 - https://community.auanet.org/policyandadvocacyblog/blogs/policy-brief/2016/11/16/coding-corner-percutaneous-nephrostolithotomy-and-nephrostomytract#:~:text=Answer%3A%C2%A0%20CPT%20code%2050080%20Percutaneous%20nephrostolithotomy%20or%20pyelostolithotomy%2C,are%20the%20base%20codes%20for%20percutaneous%20stone%20extraction.
7 - https://www.businesstimes.com.sg/startups-tech/startups/singapore-based-startups-bag-four-wins-us-investment-platform
Evangeline
NDR Medical Techology
evangeline@ndrmedical.com
Visit us on social media:
LinkedIn
YouTube
ANT-X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.