Stroke Survivor Makes Remarkable Recovery Using His Own Stem Cells
Neurologic Stem Cell Treatment Study (NEST) shows positive results in stroke; other neurologic diseases
Significant clinical improvement may be possible in different neurologic diseases using stem cells as provided in NEST”
UNITED STATES , March 30, 2023/EINPresswire.com/ -- Christopher Martin, a stroke survivor, has made a remarkable recovery following his participation in the Neurologic Stem Cell Treatment Study. The consequences of his hemorrhagic stroke in April 2016 were terrible. Martin reports: “ I pretty much went straight into atrophy and spasticity. I could hardly do anything with my right side. My shoulder was subluxed and I wore a Kafo orthosis and a shoulder brace. I hated every minute of my life”. — Steven Levy MD
Martin began to work out as much as he could in July 2019, but soon realized he needed something more to overcome the stroke. He decided to look at the latest stem cell treatment options from the Neurologic Stem Cell Treatment Study (NEST) on clinicaltrials.gov. He took part in the NEST program in October 2020. Within about 4 months he reported noticing major increases in muscle strength as the neurons damaged in the stroke began to recover and regenerate.
"I lost probably 90% of those neurons/ muscles from the stroke," Martin said. "I am now regenerating all those muscles back in 2.5-3 years. It's remarkable. Now I walk pretty normally. Everything is working better."
Martin's story is a testament to the power of neurologic advances, synergistic with exercise, to promote stroke recovery. By harnessing the stem cell fraction in NEST, he was able to jump-start his recovery, regain strength and is now much better able to walk and move.
His story is an inspiration to stroke survivors everywhere, showing that significant improvement may be possible using cutting-edge approaches in neurology. With the willingness to embrace new technology, patients suffering from different neurologic diseases may make remarkable progress toward normalcy.
WHAT IS THE TREATMENT:
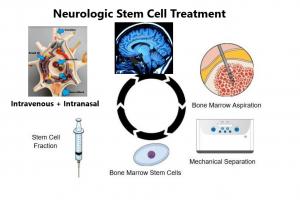
The Neurologic Stem Cell Treatment Study ( NEST) NCT02795052 uses the patient's own stem cells for treatment. The patient is provide a short period of anesthesia and there is no pain. A small amount of the patient’s own bone marrow is taken, the stem cell fraction is isolated, and the stem cells are provided intravenously and intranasally, passing readily through the circulation and bypassing the blood-brain barrier to non-invasively enter the brain and central nervous system.
OTHER NEUROLOGICAL DISEASES NEST MAY HELP:
MD Stem Cells has treated a number of neurologic diseases in NEST. Eligible conditions as listed in ClinicalTrials.gov include: Neurologic Disorders, Nervous System Diseases, Neurodegenerative Diseases, Neurological Disorders, Stroke, Traumatic Brain Injury, Cadasil, Chronic Traumatic Encephalopathy, Cerebral Infarction, Cerebral Ischemia, Cerebral Stroke, Cerebral Hemorrhage, Parkinson, Multi-System Degeneration MSA , Multiple System Atrophy, Progressive Supranuclear Palsy PSP, Amyotrophic Lateral Sclerosis ALS, Neuropathy, Diabetic Neuropathies, Alzheimer Disease, Dementia, Frontotemporal Dementia, Lewy Body Disease, Cognitive Impairment, Lewy Body Variant of Alzheimer Disease.
KEY POINTS:
Stroke, as well as other neurological diseases, may now be addressed with the NEST procedure. We have had no complications, no adverse or serious adverse events in NEST. Contact MD Stem Cells directly for a case review. MD Stem Cells the knowledge to carefully approach your neurologic condition and offer participation, if appropriate.
FOR MORE INFORMATION:
Receive information about participating in SCOTS2 by emailing Dr. Levy at stevenlevy@mdstemcells.com with your name, cell phone, email address and brief history of their disease. You may also use the contact us page on www.mdstemcells.com or call directly 203-423-9494. MD Stem Cells has no grant support and is not a pharmaceutical company; this is a patient sponsored studies and the patients pay for both treatment and travel.
Steven Levy MD
CEO, MD Stem Cells
+1 203-423-9494
stevenlevy@mdstemcells.com
Steven Levy
MD Stem Cells
+1 203-423-9494
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.