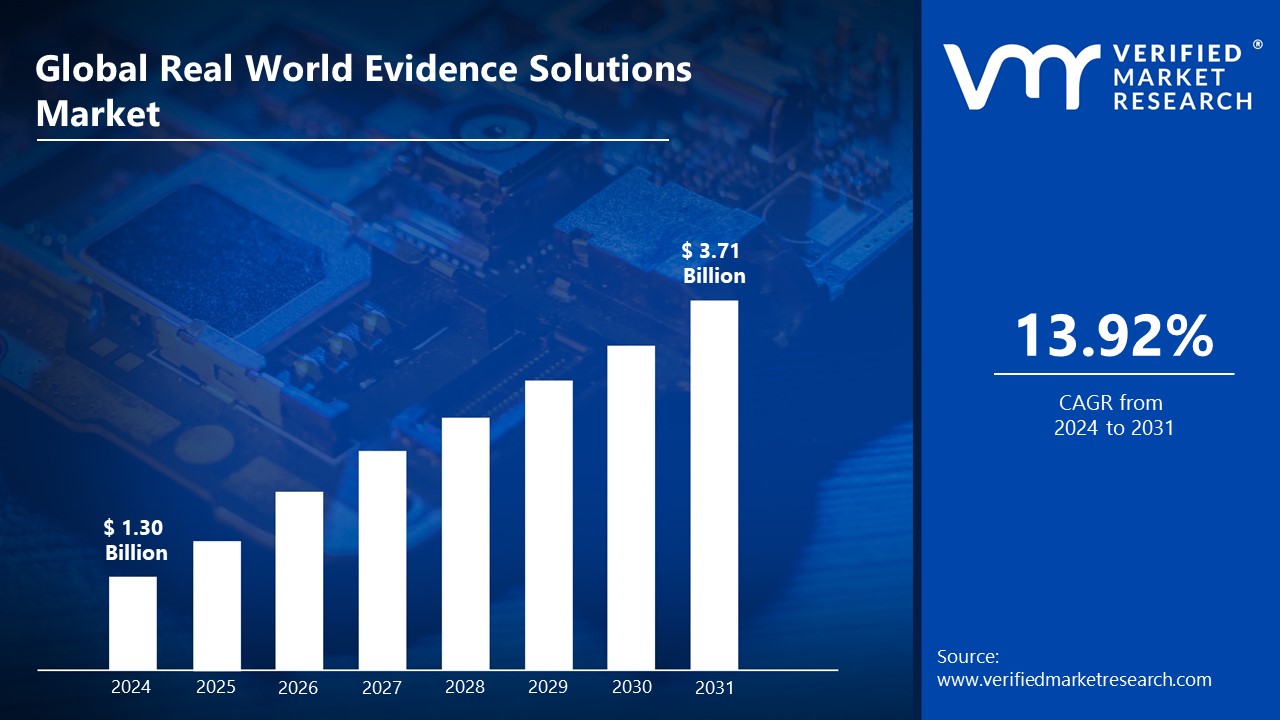
Real World Evidence Solutions Market Surges to USD 3.17 Billion by 2031, Propelled by 13.92 % CAGR - Verified Market Research®
The Real World Evidence (RWE) Solutions Market is driven by the growing need for cost-effective drug development, increasing adoption of RWE in regulatory decision-making, and rising prevalence of chronic diseases. However, market growth is restrained by concerns over data privacy, variability in data quality, and the high cost of implementing RWE solutions. Additionally, the lack of standardized methodologies poses challenges to the broader adoption of RWE solutions in the healthcare sector.
Lewes, Delaware, Aug. 26, 2024 (GLOBE NEWSWIRE) -- The Global Real World Evidence Solutions Market Size is projected to grow at a CAGR of 13.92% from 2024 to 2031, according to a new report published by Verified Market Research®. The report reveals that the market was valued at USD 1.30 Billion in 2024 and is expected to reach USD 3.71 Billion by the end of the forecast period.
Download PDF Brochure: https://www.verifiedmarketresearch.com/download-sample?rid=27946
Browse in-depth TOC on “Global Real World Evidence Solutions Market Size”
202 - Pages
126 – Tables
37 – Figures
Scope Of The Report
| REPORT ATTRIBUTES | DETAILS |
| STUDY PERIOD | 2021-2031 |
| BASE YEAR | 2024 |
| FORECAST PERIOD | 2024-2031 |
| HISTORICAL PERIOD | 2021-2023 |
| UNIT | Value (USD Million) |
| KEY COMPANIES PROFILED | IQVIA, Optum, ICON, Syneos Health, Flatiron Health, PPD, PAREXEL, Oracle, Aetion. |
| SEGMENTS COVERED | By Data Source, By Therapeutic Area, By Application, and Geography. |
| CUSTOMIZATION SCOPE | Free report customization (equivalent to up to 4 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope |
Real World Evidence Solutions Market Overview
Regulatory Support Enhancing Market Confidence: The growing endorsement of real-world evidence by regulatory agencies, such as the FDA, is driving the expansion of the Real World Evidence Solutions Market. The regulatory support incentivizes pharmaceutical companies to use Real-World Evidence (RWE) in the process of drug approvals, resulting in increased market prospects and fostering growth.
Cost-Efficient Drug Development: Real-world evidence solutions effectively decrease the duration and expenses linked to drug development by utilizing real-world data. The efficiency of Real World Evidence Solutions Market is attractive to biopharma organizations seeking to maximize their R&D efforts, making it an indispensable tool in the industry.
Rising Prevalence of Chronic Diseases: Continuous monitoring and real-time data analysis are necessary due to the increasing burden of chronic diseases worldwide. Real-world evidence solutions offer useful insights into patient outcomes, leading to their widespread adoption in the healthcare industry and contributing to the market's strong growth.

To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence?rid=27946
Data Privacy Concerns: Although the Real World Evidence Solutions Market has its benefits, it encounters difficulties as a result of strict data protection requirements. The broad adoption of RWE solutions can be hindered by concerns regarding patient data security, which can result in a slowdown of market expansion and have an influence on long-term growth.
High Implementation Costs: Smaller healthcare providers and enterprises may find it difficult to afford the expenses associated with implementing real-world evidence solutions. The exorbitant expenses associated with Real World Evidence Solutions hinder its market expansion, particularly in developing areas, and provide a substantial obstacle to the continuous progress of the industry.
Variability in Data Quality: Inconsistent and variable data quality remains a critical challenge in the Real World Evidence Solutions Market. The lack of standardized methodologies leads to unreliable insights, which can deter stakeholders from fully committing to RWE solutions, thereby restraining market growth.
Geographic Dominance:
The Real World Evidence Solutions Market is primarily dominated by North America, primarily due to its sophisticated healthcare infrastructure, significant presence of important players, and favorable regulatory environment. This dominance fosters the development of new and improved solutions in the field of renewable energy, which in turn leads to more investment and rapid expansion of the market. Moreover, the endorsement of real-world evidence by the U.S. government enhances North America's position of authority in healthcare decision-making. Nevertheless, this regional hegemony could restrict the potential for expansion in developing markets.
Real World Evidence Solutions Market Key Players Shaping the Future
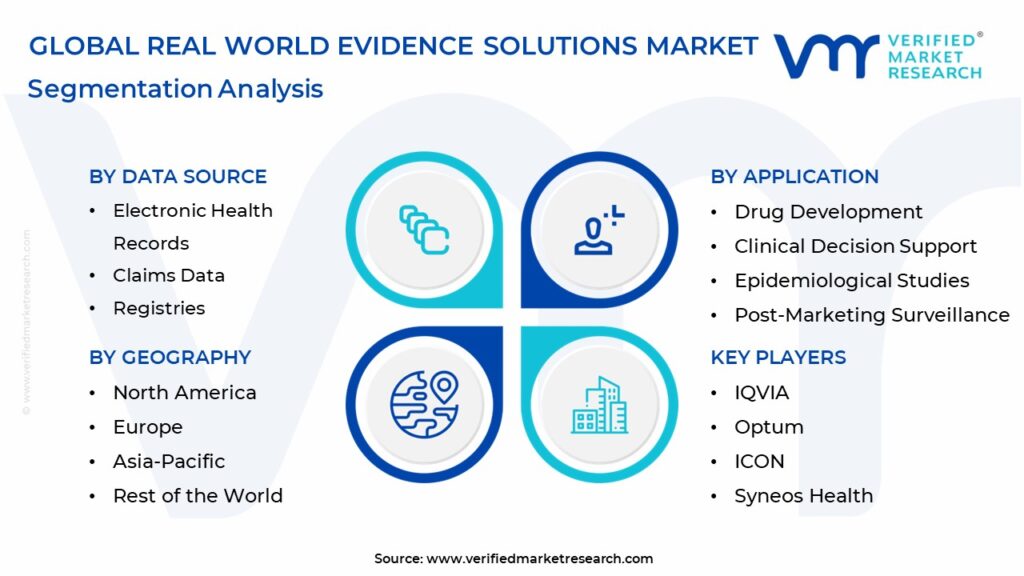
Major players, including IQVIA, Optum, ICON, Syneos Health, Flatiron Health, PPD, PAREXEL, Oracle, Aetion. and more, play a pivotal role in shaping the future of the Real World Evidence Solutions Market. Financial statements, product benchmarking, and SWOT analysis provide valuable insights into the industry's key players.
Real World Evidence Solutions Market Segment Analysis
Based on the research, Verified Market Research® has segmented the global Real World Evidence Solutions Market into Data Source, Therapeutic Area, Application, And Geography.
To get market data, market insights, and a comprehensive analysis of the Global Real World Evidence Solutions Market, please Contact Verified Market Research®.
-
Real World Evidence Solutions Market, by Data Source
- Electronic Health Records
- Claims Data
- Registries
- Medical Devices
-
Real World Evidence Solutions Market, by Therapeutic Area
- Oncology
- Cardiovascular Diseases
- Neurology
- Rare Diseases
-
Real World Evidence Solutions Market, by Application
- Drug Development
- Clinical Decision Support
- Epidemiological Studies
- Post-Marketing Surveillance
-
Real World Evidence Solutions Market, by Geography
-
North America
- U.S
- Canada
- Mexico
-
Europe
- Germany
- France
- U.K
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
-
ROW
- Middle East & Africa
- Latin America
-
North America
Browse Related Reports:
Global Laboratory Information Management System for Forensic Science Market Size By Deployment (Cloud-based LIMS, On-premise LIMS, and Remotely Hosted LIMS), By Application (Forensic Science Labs, Forensic Case Management, Forensic Medical Examiner Offices, and Forensic Property and Evidence Management), By Geography, And Forecast
Global Digital Evidence Management Market Size By Type (Cloud, On-Premises, Hybrid), Application (Law Enforcement Agencies, Others), By Geography, And Forecast
Global Tamper Evident Labels Market Size By Type (RFID Tags, Barcode), By End-Use (Food And Beverages, Cosmetic & Personal Care), By Geography, And Forecast
Global Chromatography Resin Market Size By End-User Industry (Biopharmaceutical and Pharmaceutical Industry, Food and Beverage Industry, Environmental Research and Testing, Research and Academic Institutes), By Application (Protein Purification, Drug Development and Manufacturing, Environmental Monitoring, Food and Beverage Testing), By Methods (Liquid Chromatography Resins, Gas Chromatography Resins, Resins From Affinity Chromatography, Preparative Chromatography Resins), By Geography, And Forecast
Top 7 Drug Eluting Balloon Manufacturers aiding in dose delivery
Visualize Real World Evidence Solutions Market using Verified Market Intelligence -:
Verified Market Intelligence is our BI Enabled Platform for narrative storytelling in this market. VMI offers in-depth forecasted trends and accurate Insights on over 20,000+ emerging & niche markets, helping you make critical revenue-impacting decisions for a brilliant future.
VMI provides a holistic overview and global competitive landscape with respect to Region, Country, Segment, and Key players of your market. Present your Market Report & findings with an inbuilt presentation feature saving over 70% of your time and resources for Investor, Sales & Marketing, R&D, and Product Development pitches. VMI enables data delivery In Excel and Interactive PDF formats with over 15+ Key Market Indicators for your market.
About Us
Verified Market Research® stands at the forefront as a global leader in Research and Consulting, offering unparalleled analytical research solutions that empower organizations with the insights needed for critical business decisions. Celebrating 10+ years of service, VMR has been instrumental in providing founders and companies with precise, up-to-date research data.
With a team of 500+ Analysts and subject matter experts, VMR leverages internationally recognized research methodologies for data collection and analyses, covering over 15,000 high impact and niche markets. This robust team ensures data integrity and offers insights that are both informative and actionable, tailored to the strategic needs of businesses across various industries.
VMR's domain expertise is recognized across 14 key industries, including Semiconductor & Electronics, Healthcare & Pharmaceuticals, Energy, Technology, Automobiles, Defense, Mining, Manufacturing, Retail, and Agriculture & Food. In-depth market analysis cover over 52 countries, with advanced data collection methods and sophisticated research techniques being utilized. This approach allows for actionable insights to be furnished by seasoned analysts, equipping clients with the essential knowledge necessary for critical revenue decisions across these varied and vital industries.
Verified Market Research® is also a member of ESOMAR, an organization renowned for setting the benchmark in ethical and professional standards in market research. This affiliation highlights VMR's dedication to conducting research with integrity and reliability, ensuring that the insights offered are not only valuable but also ethically sourced and respected worldwide.

Mr. Edwyne Fernandes Verified Market Research® US: +1 (650)-781-4080 US Toll Free: +1 (800)-782-1768 Email: sales@verifiedmarketresearch.com Web: https://www.verifiedmarketresearch.com/ Follow Us: LinkedIn | Twitter SOURCE – Verified Market Research®

