Enhancing the performance of proton exchange membrane water electrolysis by constructing electron/proton pathways
USA, June 24, 2024 /EINPresswire.com/ -- This research initiative entailed the development of a composite conductor capable of concurrently facilitating the conduction of protons and electrons. Functioning as a catalyst binder, the composite conductor established a dual conduction pathway for protons and electrons. In contrast to the conventional PFSA1, it demonstrated the capacity to create a more efficient active site at the three-phase interface, consequently bolstering catalytic activity and efficiency to attain superior electrochemical performance in proton exchange membrane (PEM) electrolyzers.
The proton exchange membrane electrolysis of water (PEMWE) is a critical process for hydrogen generation. However, the limited ability of electrons and protons to permeate the membrane and the inefficient arrangement of the transport structure in the catalyst layer (CL) presents significant obstacles to the widespread adoption of PEMWE.
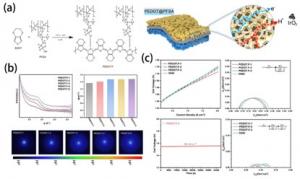
A group of researchers from China has developed a hybrid proton-electron conductor known as PEDOT:F ionomer, which has been incorporated into the CL as a catalytic binder. This development, as senior and co-corresponding author Haolin Tang explained, aimed to achieve a uniform anode CL structure and establish an effective three-phase interface by creating a proton/electron double conduction channel.
PEDOT:F, in conjunction with perfluorinated sulfonic acid (PFSA), demonstrates a high degree of side chain extensibility, promoting the formation of hydrophilic ion clusters and facilitating the adsorption of water reactants onto the catalyst's surface.
Compared to the commercial Nafion perfluorosulfonic acid (PFSA), the PEDOT:F ionomers exhibit superior oxygen evolution reaction (OER) performance as catalyst binders. According to Tang, both experimental data and Density Functional Theory (DFT) findings validate that the utilization of PEDOT enhances catalytic activity by increasing conductivity and reducing the energy barrier.
Furthermore, the enhanced electronic conductivity of PEDOT:F, combined with its larger hydrophilic ion clusters, eases the adsorption of reactant water on the catalyst's surface, promoting the electrochemical reaction. Moreover, the electrode containing PEDOT:F displayed outstanding ohmic resistance compared to that made with Nafion, with reductions of 23.4% and 17.6% at current densities of 0.1 A·cm-2 and 1.5 A·cm-2, respectively.
Tang noted that the improved performance can be attributed to the superior conductivity of both protons and electrons in PEDOT:F, along with its outstanding structural stability. This feature facilitates the smooth migration of particles during the electrolytic process, thereby enhancing the performance of PEMWE.
The researchers published their findings in the KeAi journal Advanced Powder Materials.
DOI
10.1016/j.apmate.2024.100203
Original Source URL
https://doi.org/10.1016/j.apmate.2024.100203
Funding information
This work is supported by the National Natural Science Foundation of China (52202009), Key Research and Development Program of Guangdong Province (2020B0909040001), Key R&D project of Hubei Province, China (2021AAA006), Guangdong Hydrogen Energy Institute of WHUT under Guangdong Key Areas Research and Development Program (2019B090909003).
Lucy Wang
BioDesign Research
email us here
1 https://doi.org/10.1016/j.apmate.2024.100203