FDA clears the nView s1 imaging system with integrated navigation
nView announces the 510(k) clearance of the nView s1 with navigation option by the FDA along with AI-enabled image analysis and 3D stitching
We believe that the nView s1 with integrated navigation is the most efficient solution in terms of minimizing surgical time, minimizing the footprint in the OR, and minimizing radiation to the patient”
SALT LAKE CITY, UT, UNITED STATES, November 5, 2021 /EINPresswire.com/ -- nView medical1 Inc. (nView) announces the 510(k) clearance of the nView s12 with navigation option by the U.S. Food and Drug Administration (FDA).— Cristian Atria
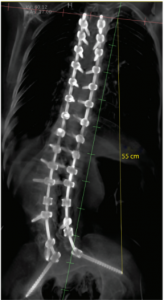
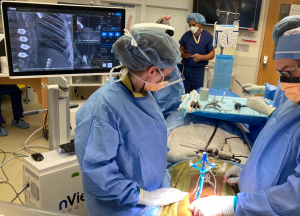
nView’s mission is to make surgery safer, faster, and consistently accurate by creating instant 3D information throughout the surgical procedure. nView s1 is an imaging system that integrates the latest developments in low-dose X-ray imaging and AI-enabled imaging algorithms to provide multiplanar, fluoroscopic and augmented views derived from fast tomographic reconstructions of the patient during surgery. With the addition of navigation these images can now be used for surgical guidance, providing greater utility for surgeons during spine procedures.
nView medical CEO, Cristian Atria, commented: “What makes nView’s approach a breakthrough is that with insta-3D™, 3D images can be taken on the fly during surgery, providing a true representation of the anatomy. These images can be navigated immediately, we call this unique approach true-map navigation™. We believe that the nView s1 with integrated navigation is the most efficient solution in terms of minimizing surgical time, minimizing the footprint in the OR, and minimizing radiation to the patient. This is a major milestone for nView as we augment our image creation offering with image utilization technologies. With this 510(k) we also cleared AI-enabled image analysis and advanced imaging modes such as 3D stitching. This further increases the value we bring to customers.”
The nView s1 with true-map navigation™ was first unveiled at the Pediatric Orthopedic Society of North America (POSNA) 2021 annual meeting. It has been successfully used in clinical cases and its use will be presented at the 15th International Congress on Early Onset Scoliosis (ICEOS)3 which will be held November 11th and 12th in Salt Lake City, UT.
nView medical, based in Salt Lake City, UT, is a startup whose mission is to make surgery safer, faster, and consistently accurate. nView develops imaging and guidance systems, bringing breakthrough AI solutions for image creation and visualization to surgery. nView medical backers include: the National Science Foundation (NSF), the National Institutes of Health (NIH), the State Of Utah, the National Consortium for Pediatric Device Innovation, HealthTech Arkansas, MedTech Innovator, Dr. Kevin Foley, and Fusion Fund.
Cristian Atria
nView medical
+1 9787128742
email us here
Visit us on social media:
LinkedIn
1 https://www.nviewmed.com
2 https://www.nviewmed.com/products
3 https://pediatricspinefoundation.org/iceos.aspx