Mesoblast Reports Financial Results and Operational Update for Fiscal Year Ended June 30, 2024
Potential first product approval and preparing for commercial launch
NEW YORK, Aug. 28, 2024 (GLOBE NEWSWIRE) -- Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today provided an operational update and reported financial results for the period ended June 30, 2024.
Mesoblast Chief Executive Silviu Itescu said: “During the past year we have built significant momentum in our interactions with the United States Food and Drug Administration (FDA) across each of our Phase 3 products.
I am very pleased that our Biologics License Application (BLA) resubmission for approval of Ryoncil® (remestemcel-L) in the treatment of children with steroid-refractory acute graft versus host disease (SR-aGVHD) was accepted by the FDA. We are in active discussions with the agency and anticipate a decision prior to or on the Prescription Drug User Fee Act (PDUFA) goal date of January 7, 2025. Concurrently we are implementing a go-to-market plan to bring RYONCIL to the many children suffering with this devastating disease, picking up the substantial amount of work completed last year.”
“We have commenced enrolling patients across multiple U.S. sites in our confirmatory Phase 3 trial for inflammatory back pain which is in alignment with FDA, we have received a Rare Pediatric Disease Designation from FDA for Revascor® (rexlemestrocel-L) in children with a congenital heart disease, and have additionally been notified that FDA supports a potential accelerated approval pathway for REVASCOR in end-stage heart failure patients.”
OPERATIONAL RESULTS FOR THE FULL-YEAR ENDED JUNE 30, 2024 (FY2024)
1. RYONCIL (REMESTEMCEL-L) FOR ACUTE GRAFT VERSUS HOST DISEASE IN CHILDREN
Potential FDA Approval
- Mesoblast resubmitted its BLA for approval of RYONCIL on July 8, 2024, addressing remaining CMC (Chemistry, Manufacturing, and Controls) items in the August 2023 Complete Response Letter (CRL).
- FDA previously informed Mesoblast that the available clinical data from its Phase 3 study appears sufficient to support resubmission of the BLA.
- FDA accepted the BLA resubmission within two weeks, considering it to be a complete response.
- Mesoblast and FDA are in ongoing interactions in relation to the active BLA review.
- FDA has already conducted the Pre-License Inspection (PLI) of the manufacturing process for RYONCIL in May 2023 and this did not result in the issuance of any Form 483.
- Mesoblast anticipates a decision prior to or on the FDA’s Prescription Drug User Fee Act (PDUFA) goal date of January 7, 2025.
Activities For Go to Market Strategy
- Hiring of select senior positions to build targeted commercial team has commenced.
- Key Pre-Launch Activities include:
- Market Access initiates payer outreach
- Medical provides education to payers
- Corporate leadership initiates engagement with Top 15 centers
- Regional sales directors lead center profiling
- Ongoing KOL engagement with greatest experience using RYONCIL at highest volume centers
- Non-promotional activities including profiling high-volume centers, education on disease awareness & unmet needs, and payer engagement
- Post-launch - Staged approach based on centers with highest volume and experience with product.
- Targeted sales force with experience in bone marrow transplant centers - 15 highest volume centers account for ~50% of patients.
2. RYONCIL (REMESTEMCEL-L) FOR ACUTE GRAFT VERSUS HOST DISEASE IN ADULTS
- Mesoblast strategy is to first gain pediatric approval for RYONCIL, followed by label extension in the larger adult population.
- As part of its label extension strategy for RYONCIL, Mesoblast is planning to conduct a study in the larger adult population once it has gained pediatric approval.
- Survival in adults with SR-aGVHD who have failed at least one additional agent, such as ruxolitinib, remains as low as 20-30% by 100 days.1,2 In contrast, 100-day survival was 67% after RYONCIL treatment was used under expanded access in 51 adults and children with SR-aGVHD who failed to respond to at least one additional agent, such as ruxolitinib.
- Mesoblast is collaborating with Blood and Marrow Transplant Clinical Trials Network (BMT CTN) in the United States, a body that is funded by the National Institutes of Health (NIH) and is responsible for approximately 80% of all US allogeneic BMTs, to conduct a pivotal trial in adults with SR-aGVHD.
3. REXLEMESTROCEL-L FOR CHRONIC LOW BACK PAIN ASSOCIATED WITH DEGENERATIVE DISC DISEASE
- The confirmatory Phase 3 trial of Mesoblast’s second generation allogeneic, STRO3-immunoselected, and industrially manufactured stromal cell product rexlemestrocel-L in patients with chronic low back pain (CLBP) due to inflammatory degenerative disc disease (DDD) of less than five years duration has commenced enrollment at multiple sites across the United States.
- FDA has previously agreed on the design of this 300-patient randomized, placebo-controlled confirmatory Phase 3 trial, and the 12-month primary endpoint of pain reduction as an approvable indication.
- This endpoint was successfully met in Mesoblast’s first Phase 3 trial.
- Key secondary measures include improvement in quality of life and function.
- A particular focus is on treatment of patients on opioids, since discogenic back pain accounts for approximately 50% of prescription opioid usage in the US.
- Significant pain reduction and opioid cessation were observed in Mesoblast’s first Phase 3 trial.
- FDA has designated rexlemestrocel-L a Regenerative Medicine Advanced Therapy (RMAT) for the treatment of chronic low back pain. RMAT designation provides all the benefits of Breakthrough and Fast Track designations, including rolling review and eligibility for priority review on filing of a BLA.
4. REVASCOR (REXLEMESTROCEL-L) FOR PEDIATRIC CONGENITAL HEART DISEASE: HYPOPLASTIC LEFT HEART SYNDROME (HLHS)
- During the year FDA granted REVASCOR both Rare Pediatric Disease Designation (RPDD) and Orphan-Drug Designation (ODD). This followed submission of results from the randomized controlled trial in children with hypoplastic left heart syndrome (HLHS), a potentially life-threatening congenital heart condition.
- Results from a blinded, randomized, placebo-controlled prospective trial of REVASCOR conducted in the United States in children with HLHS were published in the December 2023 issue of the peer reviewed The Journal of Thoracic and Cardiovascular Surgery Open (JTCVS Open).3
- In the HLHS trial conducted in 19 children, a single intramyocardial administration of REVASCOR at the time of staged surgery resulted in the desired outcome of significantly larger increases in left ventricular (LV) end-systolic and end-diastolic volumes over 12 months compared with controls as measured by 3D echocardiography, (p=0.009 & p=0.020 respectively).
- These changes are indicative of clinically important growth of the small left ventricle, facilitating the ability to have a successful surgical correction, known as full biventricular (BiV) conversion, which allows for a normal two ventricle circulation. Without full BiV conversion the right heart chamber is under excessive strain with increased risk of heart failure and death.
- On FDA approval of a BLA for REVASCOR for the treatment of HLHS, Mesoblast may be eligible to receive a Priority Review Voucher (PRV) that can be redeemed for any subsequent marketing application or may be sold or transferred to a third party.
5. REVASCOR (REXLEMESTROCEL-L) FOR CHRONIC HEART FAILURE WITH REDUCED EJECTION FRACTION (HFrEF) AND PERSISTENT INFLAMMATION
- Heart failure with low ejection fraction (HFrEF) occurs in approximately 50% of all heart failure patients and is associated with high mortality.
- Over 60% of HFrEF patients have underlying ischemia and these are at highest risk of recurrent major adverse cardiac events involving large vessels (heart attacks / strokes).
- REVASCOR has reduced major adverse cardiac events (MACE) (cardiovascular death, heart attacks and strokes) in a completed randomized controlled Phase 3 trial in ischemic HFrEF patients with NYHA class II /III disease and inflammation.
- Over 100,000 patients in the US progress annually to end-stage HFrEF and more than 2,500 life prolonging LVADs are implanted annually in these patients.
- Resistance to functional recovery in ischemic HFrEF patients with LVADs is thought to be due to excessive inflammation and microvascular insufficiency in the ischemic myocardium.4
- In a randomized controlled trial, a single administration of REVASCOR reduced inflammation, strengthened left ventricular function, reduced right ventricular failure, and reduced hospitalizations in end-stage ischemic HFrEF patients with a left ventricular assist device (LVAD).
- In March FDA informed Mesoblast that it supports an accelerated approval pathway for its second generation allogeneic, STRO3-immunoselected, and industrially manufactured stromal cell product rexlemestrocel-L (Revascor®), for patients with end-stage ischemic HFrEF and an LVAD.
- Mesoblast has received RMAT designation for rexlemestrocel-L in the treatment of end-stage heart failure in LVAD patients and intends to meet with FDA to discuss data presentation, timing and FDA expectations for an accelerated approval filing in these patients.
FINANCIAL RESULTS FOR THE FULL-YEAR ENDED JUNE 30, 2024 (FY2024)
- Cash balance at June 30, 2024 was US$63.3 million (A$95.0 million),5 with additional US$10.0 million available from an existing facility on FDA approval of RYONCIL.
- Reduction in net cash usage for operating activities:
- 23% reduction (US$14.8 million) for FY2024 compared with FY2023 (US$48.5 million vs US$63.3 million).
- 37% reduction (US$6.1 million) for Q4 FY2024 compared with Q4 FY2023 (US$10.2 million vs US$16.3 million).
- Reduction in cash usage predominantly driven by reduced manufacturing activities and lowered payroll.
Continued focus on prudent cash management for operational activities as we undertake targeted commercial rollout and supply chain activities for RYONCIL (remestemcel-L).
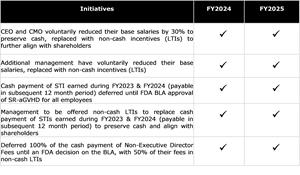
COST CONTAINMENT TARGETS ACHIEVED FOR FY2024 AND CONTINUING IN FY2025
- Achievement of 23% reduction (US$14.8 million) in net cash usage for operating activities in FY2024 was due in large part to successful execution of our payroll reduction strategy.
- Continued focus on cost containment of headcount and payroll to be maintained in FY2025.
- Alignment of management salaries and incentives with shareholders as outlined below.
DETAILS OF FINANCIAL RESULTS FOR THE TWELVE MONTHS ENDED JUNE 30, 2024 (FY2024)
- Royalties primarily on sales of TEMCELL® HS Inj.6 sold in Japan by our licensee for the FY2024 were US$5.9 million and US$6.3 million on a constant currency basis, down 17% compared with US$7.1 million for the comparative period in FY2023.7
- Research & Development expenses reduced by US$1.8 million (7%), down to US$25.4 million for FY2024 compared with US$27.2 million for the comparative period in FY2023. R&D expenses primarily supported preparations for the remestemcel-L BLA re-submission and preparations for pivotal studies for CLBP associated with DDD and adult SR-aGVHD.
- Manufacturing reduced by US$12.0 million (43%), down from US$27.7 million to US$15.7 million due to decreased inventory build and one-off FY2023 spend on FDA Pre-License Inspection (PLI).
- Management and Administration expenses reduced by US$1.7 million (7%), to US$23.6 million for FY2024.
- Revaluation of Contingent Consideration recognized in FY2024 reflects a greater probability of approval of remestemcel-L for the treatment of SR-aGVHD as compared to FY2023 which reflected the 2023 CRL. In FY2024 we recognized a loss of US$9.7 million compared to a gain of US$8.8 million in FY2023.
- Fair value movement of warrants recognized a gain of US$0.8 million in FY2024 on a revaluation of warrants to market value compared to a loss of US$2.2 million in FY2023.
- Other operating income in FY2024 was US$2.6 million compared with US$4.2 million in FY2023 due to a reduction tax incentives.
- Finance Costs for borrowing arrangements include US$17.2 million of non-cash expenditure for FY2024 comprising accruing interest and borrowing costs.
Loss after tax for FY2024 was US$88.0 million, a 7% increase compared to US$81.9 million for FY2023. The net loss attributable to ordinary shareholders was 8.91 US cents per share for FY2024, compared with 10.53 US cents per share for FY2023.
Conference Call
There will be a webcast today, beginning at 6.30pm EDT (Wednesday, August 28); 8.30am AEST (Thursday, August 29). It can be accessed via: https://webcast.openbriefing.com/msb-fyr-2024/
The archived webcast will be available on the Investor page of the Company’s website: www.mesoblast.com
About Mesoblast
Mesoblast (the Company) is a world leader in developing allogeneic (off-the-shelf) cellular medicines for the treatment of severe and life-threatening inflammatory conditions. The Company has leveraged its proprietary mesenchymal lineage cell therapy technology platform to establish a broad portfolio of late-stage product candidates which respond to severe inflammation by releasing anti-inflammatory factors that counter and modulate multiple effector arms of the immune system, resulting in significant reduction of the damaging inflammatory process.
Mesoblast has a strong and extensive global intellectual property portfolio with protection extending through to at least 2041 in all major markets. The Company’s proprietary manufacturing processes yield industrial-scale, cryopreserved, off-the-shelf, cellular medicines. These cell therapies, with defined pharmaceutical release criteria, are planned to be readily available to patients worldwide.
Mesoblast is developing product candidates for distinct indications based on its remestemcel-L and rexlemestrocel-L allogeneic stromal cell technology platforms. Remestemcel-L is being developed for inflammatory diseases in children and adults including steroid refractory acute graft versus host disease, biologic-resistant inflammatory bowel disease, and acute respiratory distress syndrome. Rexlemestrocel-L is in development for advanced chronic heart failure and chronic low back pain. Two products have been commercialized in Japan and Europe by Mesoblast’s licensees, and the Company has established commercial partnerships in Europe and China for certain Phase 3 assets.
Mesoblast has locations in Australia, the United States and Singapore and is listed on the Australian Securities Exchange (MSB) and on the Nasdaq (MESO). For more information, please see www.mesoblast.com, LinkedIn: Mesoblast Limited and Twitter: @Mesoblast
References / Footnotes
- Jagasia M et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020 May 14; 135(20): 1739–1749
- Abedin S, et al. Ruxolitinib resistance or intolerance in steroid-refractory acute graft versus-host disease — a real-world outcomes analysis. British Journal of Haematology, 2021;195:429–43.
- Wittenberg RE, Gauvreau K, Leighton J, Moleon-Shea M, Borow KM, Marx GR, Emani SM, Prospective randomized controlled trial of the safety and feasibility of a novel mesenchymal precursor cell therapy in hypoplastic left heart syndrome, JTCVS Open Volume 16, Dec 2023, doi: https://doi.org/10.1016/j.xjon.2023.09.031
- Symons JD, Deeter L, Deeter N, et al. Effect of continuous-flow left ventricular assist device support on coronary artery endothelial function in ischemic and nonischemic cardiomyopathy. Cir Heart Fail 2019; 12:e006085. DOI: 10.1161/CIRCHEARTFAILURE.119.006085.
- Using Reserve Bank of Australia (RBA) published exchange rate from June 30, 2024 of 1A$:0.6624US$.
- TEMCELL® HS Inj. is a registered trademark of JCR Pharmaceuticals Co. Ltd.
- TEMCELL sales by our Licensee are recorded in Japanese Yen before being translated into USD for the purposes of calculating the royalty paid to Mesoblast. Results have been adjusted for the movement of the USD to Japanese Yen exchange rate from 1USD:140.01 Yen for the twelve months ended June 30, 2023 to 1USD:151.75 Yen for the twelve months ended June 30, 2024.
Forward-Looking Statements
This press release includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. Forward-looking statements include, but are not limited to, statements about: the initiation, timing, progress and results of Mesoblast’s preclinical and clinical studies, and Mesoblast’s research and development programs; Mesoblast’s ability to advance product candidates into, enroll and successfully complete, clinical studies, including multi-national clinical trials; Mesoblast’s ability to advance its manufacturing capabilities; the timing or likelihood of regulatory filings and approvals (including any future decision that the FDA may make on the BLA for remestemcel-L for pediatric patients with SR-aGVHD), manufacturing activities and product marketing activities, if any; the commercialization of Mesoblast’s product candidates, if approved; regulatory or public perceptions and market acceptance surrounding the use of stem-cell based therapies; the potential for Mesoblast’s product candidates, if any are approved, to be withdrawn from the market due to patient adverse events or deaths; the potential benefits of strategic collaboration agreements and Mesoblast’s ability to enter into and maintain established strategic collaborations; Mesoblast’s ability to establish and maintain intellectual property on its product candidates and Mesoblast’s ability to successfully defend these in cases of alleged infringement; the scope of protection Mesoblast is able to establish and maintain for intellectual property rights covering its product candidates and technology; estimates of Mesoblast’s expenses, future revenues, capital requirements and its needs for additional financing; Mesoblast’s financial performance; developments relating to Mesoblast’s competitors and industry; and the pricing and reimbursement of Mesoblast’s product candidates, if approved. You should read this press release together with our risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast’s actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, and accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise.
Release authorized by the Chief Executive.
For more information, please contact:
| Corporate Communications / Investors | Media |
| Paul Hughes | BlueDot Media |
| T: +61 3 9639 6036 | Steve Dabkowski |
| E: investors@mesoblast.com | T: +61 419 880 486 |
| E: steve@bluedot.net.au | |
Consolidated Income Statement
| Year Ended June 30, | |||||
| (in U.S. dollars, in thousands, except per share amount) | 2024 | 2023 | |||
| Revenue | 5,902 | 7,501 | |||
| Research & development | (25,353 | ) | (27,189 | ) | |
| Manufacturing commercialization | (15,717 | ) | (27,733 | ) | |
| Management and administration | (23,626 | ) | (25,374 | ) | |
| Fair value remeasurement of contingent consideration | (9,693 | ) | 8,771 | ||
| Fair value remeasurement of warrant liability | 779 | (2,205 | ) | ||
| Other operating income and expenses | 2,570 | 4,250 | |||
| Finance costs | (23,009 | ) | (20,122 | ) | |
| Loss before income tax | (88,147 | ) | (82,101 | ) | |
| Income tax benefit/(expense) | 191 | 212 | |||
| Loss attributable to the owners of Mesoblast Limited | (87,956 | ) | (81,889 | ) | |
| Losses per share from continuing operations attributable to the ordinary equity holders of the Group: | Cents | Cents | |||
| Basic - losses per share | (8.91 | ) | (10.53 | ) | |
| Diluted - losses per share | (8.91 | ) | (10.53 | ) | |
Consolidated Statement of Comprehensive Income
| Year Ended June 30, | |||||
| (in U.S. dollars, in thousands) | 2024 | 2023 | |||
| Loss for the period | (87,956 | ) | (81,889 | ) | |
| Other comprehensive (loss)/income | |||||
| Items that may be reclassified to profit and loss | |||||
| Exchange differences on translation of foreign operations | 51 | (573 | ) | ||
| Items that will not be reclassified to profit and loss | |||||
| Financial assets at fair value through other comprehensive income | (743 | ) | (1 | ) | |
| Other comprehensive (loss)/income for the period, net of tax | (692 | ) | (574 | ) | |
| Total comprehensive losses attributable to the owners of Mesoblast Limited | (88,648 | ) | (82,463 | ) | |
Consolidated Balance Sheet
| As of June 30, | |||||
| (in U.S. dollars, in thousands) | 2024 | 2023 | |||
| Assets | |||||
| Current Assets | |||||
| Cash & cash equivalents | 62,960 | 71,318 | |||
| Trade & other receivables | 20,952 | 6,998 | |||
| Prepayments | 2,551 | 3,342 | |||
| Total Current Assets | 86,463 | 81,658 | |||
| Non-Current Assets | |||||
| Property, plant and equipment | 1,106 | 1,357 | |||
| Right-of-use assets | 2,732 | 5,134 | |||
| Financial assets at fair value through other comprehensive income | 1,014 | 1,757 | |||
| Other non-current assets | 2,102 | 2,326 | |||
| Intangible assets | 575,736 | 577,183 | |||
| Total Non-Current Assets | 582,690 | 587,757 | |||
| Total Assets | 669,153 | 669,415 | |||
| Liabilities | |||||
| Current Liabilities | |||||
| Trade and other payables | 7,070 | 20,145 | |||
| Provisions | 45,038 | 6,399 | |||
| Borrowings | 13,862 | 5,952 | |||
| Lease liabilities | 2,626 | 4,060 | |||
| Warrant liability | 4,647 | 5,426 | |||
| Total Current Liabilities | 73,243 | 41,982 | |||
| Non-Current Liabilities | |||||
| Provisions | 10,620 | 16,612 | |||
| Borrowings | 100,483 | 102,811 | |||
| Lease liabilities | 1,952 | 3,672 | |||
| Deferred consideration | 2,500 | 2,500 | |||
| Total Non-Current Liabilities | 115,555 | 125,595 | |||
| Total Liabilities | 188,798 | 167,577 | |||
| Net Assets | 480,355 | 501,838 | |||
| Equity | |||||
| Issued Capital | 1,310,813 | 1,249,123 | |||
| Reserves | 78,303 | 73,520 | |||
| Accumulated losses | (908,761 | ) | (820,805 | ) | |
| Total Equity | 480,355 | 501,838 | |||
Consolidated Statement of Cash Flow
| Year Ended June 30, | |||||
| (in U.S. dollars, in thousands) | 2024 | 2023 | |||
| Cash flows from operating activities | |||||
| Commercialization revenue received | 6,776 | 7,480 | |||
| Government grants and tax incentives and credits received | 3,819 | 1,118 | |||
| Payments to suppliers and employees (inclusive of goods and services tax) | (60,835 | ) | (72,683 | ) | |
| Interest received | 1,778 | 796 | |||
| Income taxes received /(paid) | 4 | 20 | |||
| Net cash (outflows) in operating activities | (48,458 | ) | (63,269 | ) | |
| Cash flows from investing activities | |||||
| Investment in fixed assets | (271 | ) | (264 | ) | |
| Receipts from investment in sublease | 234 | 120 | |||
| Payments for licenses | (60 | ) | (50 | ) | |
| Net cash (outflows) in investing activities | (97 | ) | (194 | ) | |
| Cash flows from financing activities | |||||
| Proceeds from borrowings | — | — | |||
| Repayment of borrowings | (10,000 | ) | — | ||
| Payment of transaction costs from borrowings | (1,559 | ) | (574 | ) | |
| Interest and other costs of finance paid | (5,717 | ) | (6,014 | ) | |
| Proceeds from issue of shares | 65,406 | 88,635 | |||
| Proceeds from issue of warrants | — | — | |||
| Payments for share issue costs | (4,356 | ) | (4,889 | ) | |
| Payments for lease liabilities | (3,522 | ) | (2,656 | ) | |
| Net cash inflows/(outflows) by financing activities | 40,252 | 74,502 | |||
| Net increase/(decrease) in cash and cash equivalents | (8,303 | ) | 11,039 | ||
| Cash and cash equivalents at beginning of period | 71,318 | 60,447 | |||
| FX (loss)/gain on the translation of foreign bank accounts | (55 | ) | (168 | ) | |
| Cash and cash equivalents at end of period | 62,960 | 71,318 | |||
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e713c6e7-f56f-48e2-bdb9-d8f82d7718d6