Bile Tract Cancer expansion study opens following clearance of Imugene’s MAST trial high dose cohort
- The expansion trial is expected to enrol 10 patients with bile tract cancers (cholangiocarcinoma)
- Interim results from the MAST trial have demonstrated positive responses in gastrointestinal cancers, particularly in cholangiocarcinoma where one patient treated with CF33-hNIS (VAXINIA) achieved a complete response and another patient achieved stable disease
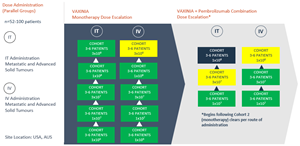
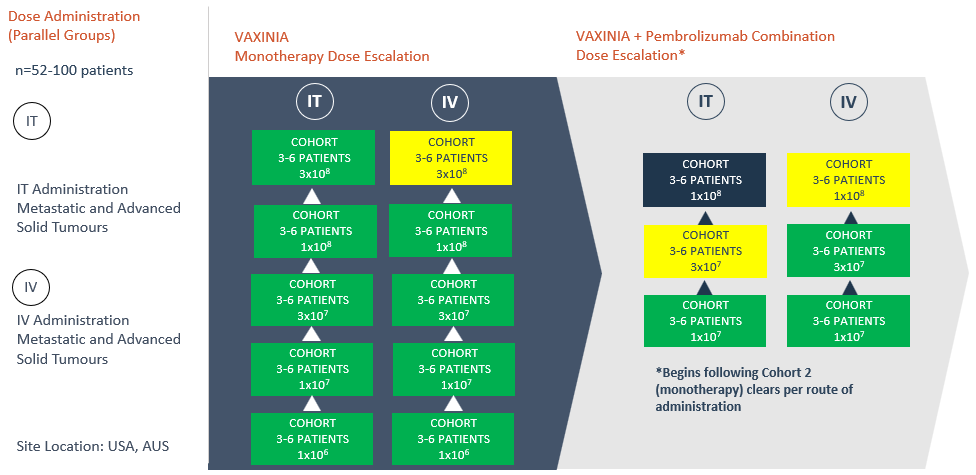
- The fifth cohort in the intratumoural (IT) arm of the VAXINIA monotherapy trial has now been cleared with no safety signals seen to date; the cohort included patients with thymic carcinoma, triple negative breast cancer and cholangiocarcinoma
SYDNEY, Australia, April 15, 2024 (GLOBE NEWSWIRE) -- Imugene Limited (ASX: IMU), a clinical stage immuno-oncology company, is pleased to announce that enrolment has opened for its expansion study in bile tract cancer (cholangiocarcinoma) patients, having completed the fifth, high dose cohort in the intratumoural (IT) arm of the monotherapy dose escalation study evaluating its cancer-killing virus CF33-hNIS (VAXINIA).
Imugene Managing Director & CEO Leslie Chong said: “As a team we’re particularly eager to begin the cholangiocarcinoma expansion study, given the meaningful difference we’ve seen VAXINIA make for patients with gastrointestinal cancers, including one patient with cholangiocarcinoma who achieved a complete response and another who achieved stable disease. It’s timely for enrolment to open as we present our VAXINIA technology to the 2024 Cholangiocarcinoma Foundation Annual Conference later this week.”
The expansion of the MAST (Metastatic Advanced Solid Tumours) Phase 1 trial is planned for 10 patients with bile tract cancers, after early positive responses were observed in gastrointestinal cancers, particularly in cholangiocarcinoma. Cholangiocarcinoma is a rare disease in which malignant cancer cells form in the bile ducts. It is difficult to treat and generally responds poorly to immunotherapy drugs.
One patient with cholangiocarcinoma who had failed three prior lines of therapy received a mid-dose of IT-administered monotherapy VAXINIA achieved a complete response, meaning the disappearance of all signs of cancer in response to treatment, with no known recurrence in more than 430 days. A second patient with cholangiocarcinoma, who has also progressed on prior drug therapies, achieved stable disease for more than four months upon receiving IV-administered VAXINIA.
In November 2023, the FDA granted the VAXINIA MAST clinical program Fast Track Designation for the treatment of bile duct cancer (cholangiocarcinoma), which allows Imugene closer cooperation with the FDA to expedite the program and potential approval process. This designation followed the promising data detailing Phase 1 efficacy and tolerability.
On Friday 12 April 2024, the Cohort Review Committee cleared the fifth cohort in the IT arm of the monotherapy dose escalation portion of the MAST trial, with no safety signals seen to date. In addition to the patients dosed in the monotherapy dose escalation portion of the trial, enrolment is ongoing for the VAXINIA and pembrolizumab combination portion of the trial, with 16 patients dosed to date.
The multicenter, Phase 1, MAST trial commenced by delivering a low dose of VAXINIA to patients with metastatic or advanced solid tumours who have had at least two prior lines of standard of care treatment. With no safety signals identified to date, the trial has since progressed through the monotherapy dose escalation cohorts as well as the combination study, whereby VAXINIA is administered with well-known checkpoint inhibitor pembrolizumab. CF33 oncolytic virus, developed by City of Hope, has been shown to shrink colon, lung, breast, ovarian and pancreatic cancer tumours in preclinical laboratory and animal models¹.
Further dose escalation to continue as long as no safety issues are observed
For more information please contact:
Leslie Chong
Managing Director and Chief Executive Officer
info@imugene.com
Investor Enquiries
shareholderenquiries@imugene.com
US Investor and Media Enquiries
Heather Armstrong
harmstrong@imugene.com
Media Enquiries
Matt Wright
matt@nwrcommunications.com.au
Connect with us on LinkedIn @Imugene Limited
Follow us on Twitter @TeamImugene
Watch us on YouTube @ImugeneLimited
References
¹ Warner SG, Kim SI, Chaurasiya S, O'Leary MP, Lu J, Sivanandam V, Woo Y, Chen NG, Fong Y. A Novel Chimeric Poxvirus Encoding hNIS Is Tumor-Tropic, Imageable, and Synergistic with Radioiodine to Sustain Colon Cancer Regression. Mol Ther Oncolytics. 2019 Apr 11;13:82-92. doi: 10.1016/j.omto.2019.04.001. PMID: 31061881; PMCID: PMC6495072.
About Imugene (ASX:IMU)
Imugene is a clinical stage immuno-oncology company developing a range of new and novel immunotherapies that seek to activate the immune system of cancer patients to treat and eradicate tumours. Our unique platform technologies seek to harness the body’s immune system against tumours, potentially achieving a similar or greater effect than synthetically manufactured monoclonal antibody and other immunotherapies. Our pipeline includes an off-the-shelf (allogeneic) cell therapy CAR T drug azer-cel (azercabtagene zapreleucel) which targets CD19 to treat blood cancers. Our pipeline also includes multiple immunotherapy B-cell vaccine candidates and an oncolytic virotherapy (CF33) aimed at treating a variety of cancers in combination with standard of care drugs and emerging immunotherapies such as CAR T’s for solid tumours. We are supported by a leading team of international cancer experts with extensive experience in developing new cancer therapies with many approved for sale and marketing for global markets.
Our vision is to help transform and improve the treatment of cancer and the lives of the millions of patients who need effective treatments. This vision is backed by a growing body of clinical evidence and peer-reviewed research. Imugene is well funded and resourced, to deliver on its commercial and clinical milestones. Together with leading specialists and medical professionals, we believe Imugene’s immuno-oncology therapies will become foundation treatments for cancer. Our goal is to ensure that Imugene and its shareholders are at the forefront of this rapidly growing global market.
Release authorised by the Managing Director and Chief Executive Officer Imugene Limited.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/24a0253d-936d-4df5-8df2-02ea3355f179