Receptor.AI helps fight obesity by acting on the “sixth taste” receptors
LONDON, UNITED KINGDOM, December 12, 2022 /EINPresswire.com/ -- A full text of the article can be found via the link2:
Obesity is one of the health problems which seems to be minor in comparison to cancer or neurodegenerative disorders from an individual perspective. However, it has huge public health implications if considered at the population level.
Putting aside metabolic and hormonal disorders, which lead to weight gain, the major cause of obesity is overeating. Usually, excessive food intake is attributed to cultural, behavioural or psychological factors, but it may also have a physiological basis.
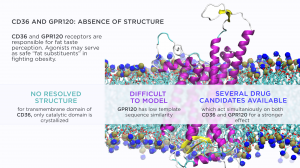
We all know from the school biology lessons that there are five basic tastes, which are sensed by dedicated receptors on our tongue: sweet, sour, bitter, salt and umami. However, recent studies identified an additional “sixth taste” devoted to the perception of dietary fatty acids. It is sensed by two receptor proteins: CD36 and GPR120, which are expressed on the taste bud cells on the tongue. Malfunctioning of these receptors was shown to cause increased intake of fatty food in mice and humans alike. Single-nucleotide polymorphism of CD36 is associated with decreased CD36 expression and decreased perception of dietary fatty acids in obese people from several ethnical groups.
These findings suggest that CD36 and GPR120 could be used as drug targets for regulating the intake of fatty food and thus fighting overeating and obesity. Agonists of these receptors may trigger a “fat-like” taste, ideally more intense than natural dietary fatty acids and trigger early satiation during eating.
A recently published paper1, co-authored by Dr Semen Yesylevskyy, the CSO of Receptor.AI, is devoted to designing drugs which can utilize this mechanism of action.
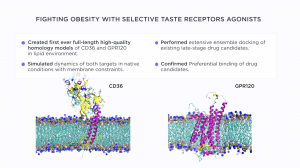
One of the challenges for rational drug development against these receptors was the absence of their full-length structures. In the case of CD36, there was no satisfactory model of the receptor membrane domain with the correct packing of its two transmembrane helices.
We created the most realistic model of CD36 to date, which includes its transmembrane region and accounts for post-translational modifications, such as multiple glycosylation sites and palmitoylation of the transmembrane helices. The dynamics of CD36 in the native membrane environment were modelled by molecular dynamics simulations to sample representative conformations of its prospective ligand binding pocket under realistic constraints imposed by the lipid membrane environment.
The GPR120 is a member of the G protein-coupled receptors family, which are also membrane proteins. The full-length homology model of GPR120 was created and simulated in the membrane environment as well to get the ensemble of representative conformations.
After that, several candidate compounds, which were previously identified by the partners, were assessed in silico by ensemble docking simulations. The usage of multiple protein conformations from MD simulations allowed for eliminating the bias, which is inevitably introduced by the single protein structure. This is especially important in the case of predicted protein structures, which don’t have a solid experimental basis in the form of X-ray, NMR or CryoEM structures.
The results of ensemble docking identified two compounds - NKS-3 and NKS-5, which are prospective agonists of CD36 and GPR120. Thorough in vitro and in vivo studies confirmed that these compounds are indeed effective on different levels: Ca2+ signalling in mouse and human taste bud cells, gustatory perception and effects on activation of the tongue-gut loop in obese mice models, and, finally, on the prevention of weight gain in mice, fed a high-fat diet.
These compounds are currently patented and are being prepared for preclinical studies in humans. However, the search for even better agonists of CD36 and GPR120 continues. Receptor.AI is collaborating with the French company EktaH, which holds the patents for NKS-3 and NKS-5, in order to develop a new generation of drug candidates for fighting obesity. In the near future, we are going to start the virtual screening project using our AI-based drug discovery platform to identify new prospective agonists of CD36 and GPR120. We’d perform both the search for better derivatives of known drug candidates and the identification of completely new molecular scaffolds with better activity and safety profiles.
Obesity is one of the health problems which seems to be minor in comparison to cancer or neurodegenerative disorders from an individual perspective. However, it has huge public health implications if considered at the population level.
Putting aside metabolic and hormonal disorders, which lead to weight gain, the major cause of obesity is overeating. Usually, excessive food intake is attributed to cultural, behavioural or psychological factors, but it may also have a physiological basis.
We all know from the school biology lessons that there are five basic tastes, which are sensed by dedicated receptors on our tongue: sweet, sour, bitter, salt and umami. However, recent studies identified an additional “sixth taste” devoted to the perception of dietary fatty acids. It is sensed by two receptor proteins: CD36 and GPR120, which are expressed on the taste bud cells on the tongue. Malfunctioning of these receptors was shown to cause increased intake of fatty food in mice and humans alike. Single-nucleotide polymorphism of CD36 is associated with decreased CD36 expression and decreased perception of dietary fatty acids in obese people from several ethnical groups.
These findings suggest that CD36 and GPR120 could be used as drug targets for regulating the intake of fatty food and thus fighting overeating and obesity. Agonists of these receptors may trigger a “fat-like” taste, ideally more intense than natural dietary fatty acids and trigger early satiation during eating.
A recently published paper1, co-authored by Dr Semen Yesylevskyy, the CSO of Receptor.AI, is devoted to designing drugs which can utilize this mechanism of action.
One of the challenges for rational drug development against these receptors was the absence of their full-length structures. In the case of CD36, there was no satisfactory model of the receptor membrane domain with the correct packing of its two transmembrane helices.
We created the most realistic model of CD36 to date, which includes its transmembrane region and accounts for post-translational modifications, such as multiple glycosylation sites and palmitoylation of the transmembrane helices. The dynamics of CD36 in the native membrane environment were modelled by molecular dynamics simulations to sample representative conformations of its prospective ligand binding pocket under realistic constraints imposed by the lipid membrane environment.
The GPR120 is a member of the G protein-coupled receptors family, which are also membrane proteins. The full-length homology model of GPR120 was created and simulated in the membrane environment as well to get the ensemble of representative conformations.
After that, several candidate compounds, which were previously identified by the partners, were assessed in silico by ensemble docking simulations. The usage of multiple protein conformations from MD simulations allowed for eliminating the bias, which is inevitably introduced by the single protein structure. This is especially important in the case of predicted protein structures, which don’t have a solid experimental basis in the form of X-ray, NMR or CryoEM structures.
The results of ensemble docking identified two compounds - NKS-3 and NKS-5, which are prospective agonists of CD36 and GPR120. Thorough in vitro and in vivo studies confirmed that these compounds are indeed effective on different levels: Ca2+ signalling in mouse and human taste bud cells, gustatory perception and effects on activation of the tongue-gut loop in obese mice models, and, finally, on the prevention of weight gain in mice, fed a high-fat diet.
These compounds are currently patented and are being prepared for preclinical studies in humans. However, the search for even better agonists of CD36 and GPR120 continues. Receptor.AI is collaborating with the French company EktaH, which holds the patents for NKS-3 and NKS-5, in order to develop a new generation of drug candidates for fighting obesity. In the near future, we are going to start the virtual screening project using our AI-based drug discovery platform to identify new prospective agonists of CD36 and GPR120. We’d perform both the search for better derivatives of known drug candidates and the identification of completely new molecular scaffolds with better activity and safety profiles.
Alan Nafiiev
Receptor.ai
+4915172837276 ext.
ai@receptor.ai
Visit us on social media:
LinkedIn
1 https://www.sciencedirect.com/science/article/pii/S2352345X22002363
2 https://www.sciencedirect.com/science/article/pii/S2352345X22002363