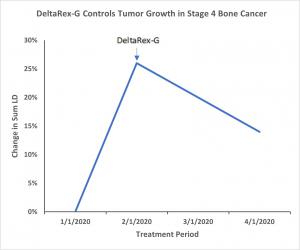
DELTAREX-G IMPEDES TUMOR GROWTH IN STAGE 4 CHEMOTHERAPY RESISTANT BONE CANCER
The spider plot graph illustrates the combined response of the patient’s lung tumors to intravenous DeltaRex-G gene therapy after failing standard chemotherapy for sarcoma.
Made possible by the Right to Try law of 2018 and the revival of a “tumor-targeted gene therapy”
DeltaRex-G is the first, and so far only, tumor-targeted genetic medicine that has been validated in the clinic worldwide. Injected intravenously, the
DeltaRex-G nanoparticles seek-out and accumulate in cancerous lesions and delivers a designer “killer” gene that sends a message to the cancer cells to self-destruct without collateral damage. DeltaRex-G has been successfully tested in 5 U.S. based and 3 Philippine-based clinical trials, which resulted in long term survival (11-12 years) of patients with hard-to-treat chemotherapy resistant Stage 4 cancers including pancreatic cancer, bone and soft tissue sarcoma, breast cancer and B-cell lymphoma (Molecular Therapy Vol 27 No 4S1 April 2019, abs 275).
The Aveni Foundation is actively raising funds for the FDA-approved “Blessed” trial in order to treat up to 40 patients. According to Dr. Erlinda Gordon, President of the Aveni Foundation: "The Aveni Foundation is deeply grateful for the Right to Try3 law of 2018, which gives cancer patients access to experimental drugs, such as DeltaRex-G, for compassionate use. To gain approval for a larger number of patients, the FDA requires an investigational drug to have demonstrated safety and efficacy in early clinical trials."
TO DONATE:
• Click on the CONTRIBUTE button at www.avenifoundation.org,
• Send a check to: Aveni Foundation, 2811 Wilshire Blvd., Suite 777, Santa Monica CA 90403, or
• Wire funds to: Chase Bank, Account Name: Aveni Foundation, Routing No: 322 271 627, Checking Acct. No.: 317 312 673, SWIFT CHASUS33
For further information, please visit our websites: www.avenifoundation.org, www.sarcomaoncology.com or contact Dr. Gordon at egordon@avenifoundation.org or egordon@sarcomaoncology.com.
Erlinda Maria Gordon
Aveni Foundation
+1 818-726-3278
egordon@avenifoundation.org
1 http://www.asgct.org/global/documents/asgct19_abstracts_-final
2 http://avenifoundation.org
3 https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/right-try


