Psoriasis Market Outlook in the 7MM Through 2034: In-Depth Analysis by Psoriasis Type | DelveInsight
The psoriasis pipeline possesses some drugs in mid- and late-stage development to be approved in the near future. The emerging landscape holds a diverse range of therapeutic alternatives for treatment, including Imsidolimab (AnaptysBio), TAK-279 (Nimbus Lakshmi/Takeda), Sonelokimab (MoonLake Immunotherapeutics), and others in different lines of treatment. The expected launch of these therapies shall further positively impact the market.
New York, USA, April 14, 2025 (GLOBE NEWSWIRE) -- Psoriasis Market Outlook in the 7MM Through 2034: In-Depth Analysis by Psoriasis Type | DelveInsight
The psoriasis pipeline possesses some drugs in mid- and late-stage development to be approved in the near future. The emerging landscape holds a diverse range of therapeutic alternatives for treatment, including Imsidolimab (AnaptysBio), TAK-279 (Nimbus Lakshmi/Takeda), Sonelokimab (MoonLake Immunotherapeutics), and others in different lines of treatment. The expected launch of these therapies shall further positively impact the market.
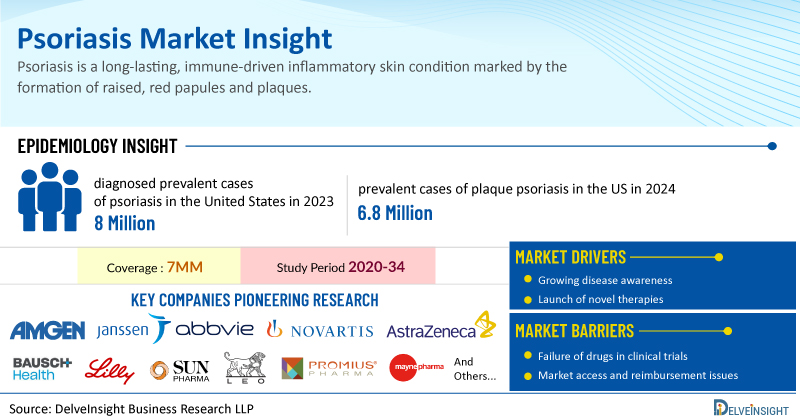
Psoriasis is a long-lasting, immune-driven inflammatory skin condition marked by the formation of raised, red papules and plaques. It results from an overactive immune response that accelerates skin cell turnover and triggers inflammation. Globally, psoriasis affects approximately 1% to 8% of the population, with about one-third of cases starting in childhood.
In 2023, the estimated number of diagnosed prevalent cases of psoriasis in the United States was around 8 million. While there is currently no cure, various effective treatments are available. Topical therapy remains the standard of care, especially for mild to moderate cases. These cases are often managed with topical glucocorticoids, vitamin D analogs, and phototherapy. In contrast, moderate to severe psoriasis typically requires systemic therapy.
Topical treatments are usually the first choice in managing psoriasis as they help slow down rapid skin cell growth and reduce associated inflammation. The introduction of biologic therapies has significantly improved the management of plaque psoriasis.
Looking ahead, the psoriasis treatment landscape is expected to expand with the introduction of new therapies from key players such as AnaptysBio, MoonLake Immunotherapeutics, Takeda, Evelo Biosciences, UNION Therapeutics, and others, potentially driving growth in the psoriasis market.
Discover more about the psoriasis market in detail @ Psoriasis Market Report
DelveInsight has expertise in the autoimmune diseases market, and an experienced team handles the neurological disorders domain proficiently. DelveInsight has recently released a series of epidemiology-based market reports on different types of psoriasis including Plaque Psoriasis, Generalized Pustular Psoriasis, Mild to Moderate Plaque Psoriasis, and Moderate to Severe Psoriasis. These reports include a comprehensive understanding of current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034 segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Additionally, the reports feature an examination of prominent companies working with their lead candidates in different stages of clinical development. Let’s dive deeply into the market assessment of these psoriasis types individually.
Plaque psoriasis is the most common form of psoriasis. These plaques typically appear on the scalp, elbows, knees, and lower back but can affect any part of the body. The condition results from an accelerated skin cell lifecycle triggered by immune system dysfunction, particularly involving T-cell activation and cytokine release. Though not contagious, plaque psoriasis significantly impacts quality of life, causing itching, pain, and emotional distress. Its exact cause is unknown but is believed to be a combination of genetic and environmental factors.
The most common form of psoriasis is chronic plaque psoriasis, affecting about 90% of psoriasis patients, with about 20%–30% of them suffering from a moderate or severe condition. In 2024, the United States accounted for around 6.8 million prevalent cases of plaque psoriasis. These cases are expected to increase in the forecast period (2025-2034).
Treatment for plaque psoriasis depends on the severity and includes topical agents, phototherapy, and systemic therapies. Topical treatments like corticosteroids, vitamin D analogs, and calcineurin inhibitors are typically used for mild cases. Biologic drugs have been a significant advancement in the treatment of plaque psoriasis. These drugs target specific parts of the immune system involved in the development of psoriasis, offering effective control of symptoms for many patients. Drugs such as adalimumab, etanercept, ustekinumab, secukinumab, and ixekizumab have been among the top players in this segment.
Additionally, some of the drugs in the pipeline include ME3183 (Meiji Seika Pharma), SGX302 (Soligenix), TAK-279 (Takeda), and others. DelveInsight’s analysts estimate that the chronic plaque psoriasis market is poised to show significant growth, mainly attributed to increasing prevalence, recent drug approvals, and anticipated launch of novel therapies during the forecast period (2024–2034).
For a comprehensive view of the plaque psoriasis market, check out the Plaque Psoriasis Market Assessment
Generalized Pustular Psoriasis Market
Generalized pustular psoriasis (GPP), a rare and severe form of pustular psoriasis, is marked by sudden, recurring flare-ups that include high-grade fever, widespread red and pustular skin eruptions, and painful, sometimes disfiguring skin symptoms, often accompanied by systemic signs resembling sepsis.
While the condition most commonly appears between the ages of 40 and 59, cases in infants and children have also been documented. In 2023, the estimated number of people living with pustular psoriasis in the United States was approximately 240,000, with projections indicating a likely increase through 2034. SPEVIGO (spesolimab) became the first FDA-approved treatment for GPP in the US in 2022. In Japan, BIMZELX (bimekizumab) was also approved for the treatment of GPP in the same year.
Currently, the GPP treatment pipeline remains limited, with only one drug—imsidolimab from AnaptysBio—being evaluated in a Phase III trial specifically for this indication. Imsidolimab works by directly blocking the interleukin-36 receptor (IL-36R), a critical pathway in the inflammatory processes driving GPP. The dynamics of the generalized pustular psoriasis market are expected to shift in the coming years due to the increasing healthcare spending worldwide and the rising prevalence of GPP.
Discover more about generalized pustular psoriasis drugs in development @ Generalized Pustular Psoriasis Clinical Trials
Mild to Moderate Plaque Psoriasis Market
Plaque psoriasis commonly manifests as large, oval or circular plaques on areas such as the scalp, trunk, and extensor surfaces of the body. These plaques are typically covered with thick scales due to rapid epidermal cell turnover.
Around 15% of individuals with plaque psoriasis are likely to develop psoriatic arthritis over time. Diagnosis is generally made through a physical examination of the skin, a review of medical history, and in some cases, a biopsy or blood tests to exclude other conditions.
Currently, OTEZLA (apremilast) is the only approved medication specifically indicated for treating mild to moderate plaque psoriasis. OTEZLA is an oral small-molecule drug that selectively inhibits phosphodiesterase 4 (PDE4), an enzyme involved in breaking down cyclic adenosine monophosphate (cAMP). By blocking PDE4, OTEZLA increases intracellular cAMP levels, which is believed to help regulate the production of inflammatory mediators. Although the exact mechanisms behind its therapeutic effects remain unclear, OTEZLA has shown benefits in managing inflammation-related conditions.
In December 2021, the US FDA expanded OTEZLA's approval to include adult patients with mild to moderate plaque psoriasis who are suitable candidates for phototherapy or systemic treatment, broadening its indication beyond moderate to severe cases.
Several late-stage investigational treatments, including IRX4204 and SGX302, are in development. The anticipated market entry of these and other emerging therapies between 2025 and 2034 is expected to positively influence the growth of the mild to moderate plaque psoriasis market in the US.
To gain a deeper understanding of the mild to moderate plaque psoriasis market, be sure to explore the Mild to Moderate Plaque Psoriasis Market Outlook
Moderate to Severe Psoriasis Market
Moderate to severe psoriasis is a more extensive and impactful form of the chronic autoimmune skin condition, psoriasis, characterized by widespread plaques affecting more than 3–10% of the body surface area or causing significant life impairment. In these cases, the inflammation goes beyond cosmetic concern, often accompanied by systemic symptoms and a higher likelihood of developing psoriatic arthritis, cardiovascular disease, metabolic syndrome, and mental health issues such as anxiety and depression. Patients with moderate to severe psoriasis require systemic treatments due to the scale and severity of the disease.
The global prevalence of moderate to severe psoriasis is estimated at around 0.3% to 1% of the population, with a higher burden in North America and Europe. While mild psoriasis accounts for the majority of cases, about 20–30% of psoriasis patients fall into the moderate to severe category. These patients are often candidates for phototherapy, traditional systemic agents like methotrexate, cyclosporine, and acitretin, or biologics that target specific inflammatory pathways. Treatment selection depends on the patient’s disease severity, comorbidities, and response to previous therapies.
Approved therapies for moderate to severe psoriasis have expanded dramatically over the past decade. Biologics have become the gold standard for many patients, offering targeted action against inflammatory cytokines such as TNF-alpha (e.g., adalimumab, etanercept), IL-12/23 (ustekinumab), IL-17 (secukinumab, ixekizumab, brodalumab), and IL-23 (guselkumab, risankizumab, tildrakizumab). Additionally, newer oral small molecules like deucravacitinib, a TYK2 inhibitor, have shown promise due to their convenience and strong efficacy profiles.
Leading pharmaceutical companies in this space include AbbVie, Novartis, Eli Lilly, Janssen (J&J), Amgen, and Bristol Myers Squibb, all of which have invested heavily in biologics and targeted oral therapies. The market for moderate to severe psoriasis is expected to continue its growth, driven by increasing biologic penetration, new entrants in the TYK2 and JAK inhibitor classes, and a growing focus on patient stratification and personalized treatment regimens.
Explore in-depth for a comprehensive understanding of the Moderate to Severe Clinical Trials
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

