Advanced Urothelial Carcinoma Clinical Trial Pipeline Analysis Demonstrates 20+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
The advanced urothelial carcinoma market is witnessing significant growth, driven by an aging population and the rising prevalence of metastatic cases. Advances in immunotherapy and targeted treatments, such as checkpoint inhibitors and novel molecular targets, are transforming the treatment landscape with more personalized options. Increased research funding and ongoing clinical trials accelerate drug development, further expanding market opportunities. Additionally, the growing adoption of immunotherapies and precision medicine continues to shape the competitive dynamics of the advanced urothelial cancer drug market.
New York, USA, March 03, 2025 (GLOBE NEWSWIRE) -- Advanced Urothelial Carcinoma Clinical Trial Pipeline Analysis Demonstrates 20+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
The advanced urothelial carcinoma market is witnessing significant growth, driven by an aging population and the rising prevalence of metastatic cases. Advances in immunotherapy and targeted treatments, such as checkpoint inhibitors and novel molecular targets, are transforming the treatment landscape with more personalized options. Increased research funding and ongoing clinical trials accelerate drug development, further expanding market opportunities. Additionally, the growing adoption of immunotherapies and precision medicine continues to shape the competitive dynamics of the advanced urothelial cancer drug market.
DelveInsight’s 'Advanced Urothelial Carcinoma Pipeline Insight 2025' report provides comprehensive global coverage of pipeline advanced urothelial carcinoma therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the advanced urothelial carcinoma pipeline domain.
Key Takeaways from the Advanced Urothelial Carcinoma Pipeline Report
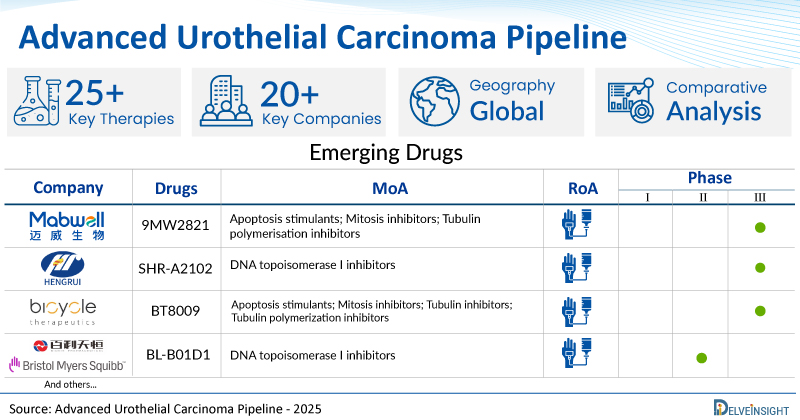
- DelveInsight’s advanced urothelial carcinoma pipeline report depicts a robust space with 20+ active players working to develop 25+ pipeline advanced urothelial carcinoma drugs.
- Key advanced urothelial carcinoma companies such as Jiangsu Hengrui Pharmaceuticals Co., Ltd., Evopoint Biosciences Inc., Bicycle Therapeutics, Bristol-Myers Squibb, Sichuan Baili Pharmaceutical Co., Ltd., BeiGene, Aurigene Discovery Technologies Limited, Mabwell (Shanghai) Bioscience Co., Ltd., Tyra Biosciences, Inc, and others are evaluating new advanced urothelial carcinoma drugs to improve the treatment landscape.
- Promising pipeline advanced urothelial carcinoma therapies such as SHR-A2102, XNW5004, BT8009, BL-B01D1, BGB-A445, AUR106, 9MW2821, TYRA-300, and others are under different phases of advanced urothelial carcinoma clinical trials.
- In February 2025, Phase I results for SHR-A2102 in patients with advanced or metastatic urothelial carcinoma were published in the 2025 ASCO Genitourinary Cancers Symposium.
- In December 2024, the Center for Drug Evaluation (CDE) of China's National Medical Products Administration announced that Jiangsu Hengrui Pharmaceuticals Co., Ltd.' SHR-A2102 wass proposed to be included as a breakthrough therapy, indicated for monotherapy for locally advanced or metastatic urothelial carcinoma failing prior platinum-based chemotherapy and PD-(L)1 inhibitor treatment.
- In September 2024, Phase II results for BL-B01D1 were presented at the 2024 ESMO Congress. Results demonstrated preliminary efficacy and safety in patients with previously treated locally advanced or metastatic urothelial carcinoma.
- In August 2024, Mabwell announced its submission to the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) for Phase III clinical study of 9MW2821 in combination with Toripalimab versus standard chemotherapy in first-line locally advanced or metastatic urothelial cancer was approved.
- In January 2023, BT8009 received Fast Track Designation (FTD) as monotherapy for adults with previously treated locally advanced or metastatic urothelial carcinoma.
Request a sample and discover the recent advances in advanced urothelial carcinoma drugs @ Advanced Urothelial Carcinoma Pipeline Report
The advanced urothelial carcinoma pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage advanced urothelial carcinoma drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the advanced urothelial carcinoma clinical trial landscape.
Advanced Urothelial Carcinoma Overview
Advanced urothelial carcinoma, also known as transitional cell carcinoma, is an aggressive bladder cancer originating in the urothelium, the tissue lining the bladder and parts of the urinary tract. Accounting for over 90% of bladder cancers, it has a high recurrence rate, with nearly 70% of patients experiencing a relapse within five years of initial treatment. Advanced urothelial carcinoma often presents as muscle-invasive or metastatic disease, which poses significant challenges in management and prognosis. Standard treatment typically involves cisplatin-based chemotherapy regimens, which show response rates of up to 70% in some patients. However, these treatments are rarely curative, and recurrence is common. Recent advances in molecular research have driven the development of targeted therapies and immunotherapies, providing new hope for improved outcomes in advanced urothelial carcinoma patients.
The causes of advanced urothelial carcinoma are multifactorial, involving genetic, environmental, and lifestyle factors. Tobacco use is a major risk factor, as smokers are two to six times more likely to develop bladder cancer compared to non-smokers. Occupational exposure to chemicals like aromatic amines, aniline dyes, and industrial solvents also increases risk. Chronic bladder irritation or inflammation, often from recurrent urinary tract infections, catheter use, or conditions such as schistosomiasis, can lead to cellular changes that predispose individuals to cancer. Genetic mutations, particularly in FGFR3, TP53, and RAS genes, play a critical role in UC pathogenesis.
Advanced urothelial carcinoma often presents with symptoms that impact patients’ quality of life. Hematuria is the most common symptom, affecting 80–90% of patients and appearing as either visible or microscopic blood. Irritative bladder symptoms, such as painful urination, frequent urination, and urgency, are also common. As the disease advances, symptoms may include lower back pain, pelvic pain, and abdominal discomfort, suggesting metastasis or local invasion. Systemic symptoms like fatigue, unintended weight loss, and malaise often indicate advanced or metastatic disease. Distant metastases may cause bone pain or leg swelling from lymphatic obstruction or deep vein thrombosis. Ureteral obstruction, leading to renal colic or hydronephrosis, can further complicate the clinical picture.
The management of advanced urothelial carcinoma has evolved significantly, incorporating chemotherapy, immunotherapy, and targeted therapies to enhance patient outcomes. Platinum-based chemotherapy, particularly cisplatin combined with gemcitabine, remains the first-line treatment, demonstrating strong efficacy. For cisplatin-ineligible patients, carboplatin-based regimens are commonly used. Immune checkpoint inhibitors (ICIs) have transformed the treatment landscape, with PD-1 and PD-L1 inhibitors proving effective in first-line settings and as maintenance therapy post-chemotherapy. Avelumab, an anti-PD-L1 antibody, has been approved as maintenance therapy for patients without disease progression after initial treatment, highlighting the progress in advanced urothelial carcinoma treatment.
Find out more about advanced urothelial carcinoma drugs @ Advanced Urothelial Carcinoma Analysis
A snapshot of the Pipeline Advanced Urothelial Carcinoma Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| 9MW2821 | Mabwell (Shanghai) Bioscience Co., Ltd. | III | Apoptosis stimulants; Mitosis inhibitors; Tubulin polymerisation inhibitors | Intravenous |
| SHR-A2102 | Jiangsu Hengrui Pharmaceuticals Co., Ltd. | III | DNA topoisomerase I inhibitors | Intravenous |
| BT8009 | Bicycle Therapeutics | II/III | Apoptosis stimulants; Mitosis inhibitors; Tubulin inhibitors; Tubulin polymerization inhibitors | Intravenous |
| BL-B01D1 | Sichuan Baili Pharmaceutical Co., Ltd./Bristol Myers Squibb | II | DNA topoisomerase I inhibitors | Intravenous |
| BGB-A445 | BeiGene | I/II | Antibody-dependent cell cytotoxicity; OX40 receptor agonists; T lymphocyte stimulants | Intravenous |
| AUR106 | Aurigene Discovery Technologies Limited | I | Programmed cell death-1 ligand-1 inhibitors; TIGIT protein inhibitors | Oral |
Learn more about the emerging advanced urothelial carcinoma therapies @ Advanced Urothelial Carcinoma Clinical Trials
Advanced Urothelial Carcinoma Therapeutics Assessment
The advanced urothelial carcinoma pipeline report proffers an integral view of the emerging advanced urothelial carcinoma therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Advanced Urothelial Carcinoma Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Apoptosis stimulants, Mitosis inhibitors, Tubulin polymerization inhibitors, Programmed cell death 1 receptor antagonists, DNA topoisomerase I inhibitors, Programmed cell death-1 ligand-1 inhibitors, TIGIT protein inhibitors, Antibody-dependent cell cytotoxicity, OX40 receptor agonists, T lymphocyte stimulants
- Key Advanced Urothelial Carcinoma Companies: Jiangsu Hengrui Pharmaceuticals Co., Ltd., Evopoint Biosciences Inc., Bicycle Therapeutics, Bristol-Myers Squibb, Sichuan Baili Pharmaceutical Co., Ltd., BeiGene, Aurigene Discovery Technologies Limited, Mabwell (Shanghai) Bioscience Co., Ltd., Tyra Biosciences, Inc, and others.
- Key Advanced Urothelial Carcinoma Pipeline Therapies: SHR-A2102, XNW5004, BT8009, BL-B01D1, BGB-A445, AUR106, 9MW2821, TYRA-300, and others.
Dive deep into rich insights for new advanced urothelial carcinoma treatments, visit @ Advanced Urothelial Carcinoma Drugs
Table of Contents
| 1. | Advanced Urothelial Carcinoma Pipeline Report Introduction |
| 2. | Advanced Urothelial Carcinoma Pipeline Report Executive Summary |
| 3. | Advanced Urothelial Carcinoma Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Advanced Urothelial Carcinoma Clinical Trial Therapeutics |
| 6. | Advanced Urothelial Carcinoma Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Advanced Urothelial Carcinoma Pipeline: Late-Stage Products (Phase III) |
| 8. | Advanced Urothelial Carcinoma Pipeline: Mid-Stage Products (Phase II) |
| 9. | Advanced Urothelial Carcinoma Pipeline: Early-Stage Products (Phase I) |
| 10. | Advanced Urothelial Carcinoma Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Advanced Urothelial Carcinoma Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Advanced Urothelial Carcinoma Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the advanced urothelial carcinoma pipeline therapeutics, reach out @ Advanced Urothelial Carcinoma Therapeutics
Related Reports
Urothelial Carcinoma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key urothelial carcinoma companies including Pfizer, Merck, Eisai Inc, AstraZeneca, Seagen Inc, Bayer, Incyte Corporation, Acerta Pharma BV, among others.
Urothelial Carcinoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key urothelial carcinoma companies, including MedPacto, AstraZeneca, Helsinn, QED Therapeutics, Inovio Pharmaceuticals, Abbisko Therapeutics, Bayer, 4D pharma plc, RemeGen, Infinity Pharmaceuticals, Kyowa Kirin, Inc., Ikena Oncology, Vyriad, Seagen, RemeGen, Pfizer, Incyte Corporation, Prestige BioPharma, TARIS Biomedical, Janssen Research and Development, among others.
Metastatic Urothelial Carcinoma Market
Metastatic Urothelial Carcinoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key metastatic urothelial carcinoma companies including Novaliq GmbH, Kowa, D. Western Therapeutics Institute, Sun Pharma Advanced Research Company, among others.
Metastatic Urothelial Carcinoma Pipeline
Metastatic Urothelial Carcinoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key metastatic urothelial carcinoma companies, including Ectin Research AG, Eli Lilly and Company, Astellas Pharma Inc, Seagen Inc., Shanghai Miracogen Inc., Advaxis, Inc., 4D pharma plc, Taiho Oncology, Shanghai Miracogen Inc., Exelixis, AstraZeneca, Incyte Corporation, IO Biotech, Jazz Pharmaceuticals, Ectin Research AB, Inovio Pharmaceuticals, Janssen Research & Development, LLC, Sumitomo Pharma Oncology, Bayer, Nurix Therapeutics, Ikena Oncology, XNK Therapeutics AB, Nektar Therapeutics, Tyra Biosciences, Inc., ALX Oncology, Scholar Rock, Inc., Jounce Therapeutics, Inc., among others.
Bladder Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key bladder cancer companies, including Pfizer, Celgene Corporation, Eli Lilly, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Sanofi, Hoffmann-La Roche, Novartis International, Johnson & Johnson, Merck & Co Inc., Vault Pharma Inc., Vyriad Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

