JAK Inhibitors Clinical Trial Pipeline Appears Robust With 50+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight | DelveInsight
JAK inhibitors are revolutionizing the treatment landscape across multiple diseases, from autoimmune disorders to cancer. By precisely targeting the JAK-STAT pathway, these small molecules disrupt aberrant signaling that fuels inflammation and tumor progression. As research uncovers new applications, JAK inhibitors stand at the forefront of next-generation therapies, unlocking unprecedented potential in immunology and oncology.
New York, USA, March 03, 2025 (GLOBE NEWSWIRE) -- JAK Inhibitors Clinical Trial Pipeline Appears Robust With 50+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight | DelveInsight
JAK inhibitors are revolutionizing the treatment landscape across multiple diseases, from autoimmune disorders to cancer. By precisely targeting the JAK-STAT pathway, these small molecules disrupt aberrant signaling that fuels inflammation and tumor progression. As research uncovers new applications, JAK inhibitors stand at the forefront of next-generation therapies, unlocking unprecedented potential in immunology and oncology.
DelveInsight’s 'JAK Inhibitors Pipeline Insight 2025' report provides comprehensive global coverage of pipeline JAK inhibitors in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the JAK inhibitors pipeline domain.
Key Takeaways from the JAK Inhibitors Pipeline Report
- DelveInsight’s JAK inhibitors pipeline report depicts a robust space with 50+ active players working to develop 55+ pipeline JAK inhibitors.
- Key JAK inhibitors companies such as Incyte Corporation, Aclaris Therapeutics, Sareum, Takeda, AstraZeneca, Ajax Therapeutics, Pfizer, GSK, Dizal Pharmaceutical, Confluence Life Sciences, Celon Pharma, Arcutis Biotherapeutics, Reistone Biopharma, and others are evaluating new JAK inhibitors drugs to improve the treatment landscape.
- Promising pipeline JAK inhibitors such as Povorcitinib, CPL409116, ATI-2138, SDC 1802, Zasocitinib, AZD 4604, INCB-160058, Research programme: JAK2 inhibitors, Ritlecitinib, Momelotinib, DZD4205, ATI 2138, CPL 409116, Itacitinib, Ivarmacitinib, and others are under different phases of JAK inhibitors clinical trials.
- In October 2024, Pelabresib in combination with ruxolitinib showed benefit in treating patients with JAK Inhibitor-Naïve Myelofibrosis significantly reduced splenomegaly, and improved anemia that has not been previously treated with Janus kinase inhibitors (JAKis).
- In September 2024, Eli Lilly and Company and EVA Pharma announced that the companies have agreed to expand access to baricitinib to an estimated 20,000 people in 49 low- to middle-income countries in Africa by 2030.
Request a sample and discover the recent advances in JAK inhibitors drugs @ JAK Inhibitors Pipeline Report
The JAK inhibitors pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage JAK inhibitors drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the JAK inhibitors clinical trial landscape.
JAK Inhibitors Overview
Janus kinase (JAK) inhibitors are small molecules, typically around 400 Da in size, that can be administered orally. JAKs are phosphotransferase enzymes that associate with the intracellular regions of cytokine receptors, facilitating signal transmission to activate immune responses. Cytokines that utilize JAKs for signaling include various interleukins, interferons, colony-stimulating factors, and hormone-like cytokines such as erythropoietin. These cytokines interact with receptors that signal through different combinations of four JAKs—JAK1, JAK2, JAK3, and TYK2.
First-generation JAK inhibitors, also known as jakinibs, such as tofacitinib and baricitinib (as well as oclacitinib for canine use), inhibit multiple JAKs simultaneously, thereby blocking a broad spectrum of cytokines. These pan-JAK inhibitors are being explored as potential treatments for numerous autoimmune conditions.
The JAK-STAT signaling pathway is employed by type I and II cytokine receptors, along with receptors for interferons and growth factors. Since these receptors lack intrinsic enzymatic activity, they rely on JAKs for downstream signaling and subsequent gene expression regulation. The name "Janus" is derived from the Roman god of doorways, symbolizing JAKs' role in transmitting signals from the cell surface to the interior. Each cytokine receptor is typically associated with a specific pair of JAKs, often forming heterodimers.
Upon cytokine binding, the receptors undergo cross-linking, triggering transphosphorylation between the associated JAKs. These activated JAKs then phosphorylate the receptor’s intracellular tail, creating a docking site for STAT (Signal Transducer and Activator of Transcription) proteins, which are normally present in the cytoplasm. The JAKs phosphorylate these STATs, prompting them to dissociate from the receptor, form homo- or heterodimers, and migrate to the nucleus, where they function as transcription factors to regulate gene expression. In mammals, there are seven STAT proteins, each associated with distinct signaling pathways, similar to JAKs.
Dysregulation of JAK activity—whether through increased or reduced kinase function—can contribute to various pathological conditions, including immunodeficiencies, inflammatory disorders, hematological abnormalities, autoimmune diseases, myeloproliferative syndromes, and increased susceptibility to infections. JAK dysregulation can result from gene translocations, inherited or somatic point mutations, receptor alterations, or changes in regulatory proteins such as SOCS or phosphatases.
The development of JAK inhibitors has revolutionized therapeutic approaches for a wide range of conditions, from malignancies to autoimmune diseases. Initially introduced to treat rare hematologic disorders, these inhibitors are now expanding into broader applications, including cancer and common autoimmune diseases.
Given the strong connection between JAK activity and various diseases, there is growing interest in designing JAK-specific inhibitors for treating myeloproliferative disorders and cancers or as immunosuppressive agents. Advances in structural biology have significantly contributed to the rational design of these inhibitors, and in recent years, crystal structures of all enzymatically active JAK kinase domains have been elucidated in complex with various ATP-competitive inhibitors.
Find out more about JAK inhibitors drugs @ JAK Inhibitors Analysis
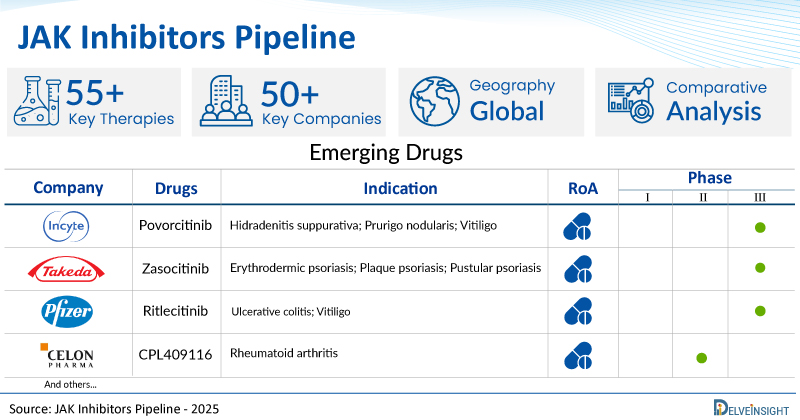
A snapshot of the Pipeline JAK Inhibitors Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| Povorcitinib | Incyte Corporation | III | Hidradenitis suppurativa; Prurigo nodularis; Vitiligo | Oral |
| Zasocitinib | Takeda | III | Erythrodermic psoriasis; Plaque psoriasis; Pustular psoriasis | Oral |
| Ritlecitinib | Pfizer03 | III | Ulcerative colitis; Vitiligo | Oral |
| CPL409116 | Celon Pharma | II | Rheumatoid arthritis | Oral |
| ATI-2138 | Aclaris Therapeutics | II | Atopic dermatitis | Oral |
| INCB-160058 | Incyte Corporation | I | Myeloproliferative disorders | Oral |
Learn more about the emerging JAK inhibitors @ JAK Inhibitors Clinical Trials
JAK Inhibitors Therapeutics Assessment
The JAK inhibitors pipeline report proffers an integral view of the emerging JAK inhibitors segmented by stage, product type, molecule type, and route of administration.
Scope of the JAK Inhibitors Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Key JAK Inhibitors Companies: Incyte Corporation, Aclaris Therapeutics, Sareum, Takeda, AstraZeneca, Ajax Therapeutics, Pfizer, GSK, Dizal Pharmaceutical, Confluence Life Sciences, Celon Pharma, Arcutis Biotherapeutics, Reistone Biopharma, and others
- Key Pipeline JAK Inhibitors: Povorcitinib, CPL409116, ATI-2138, SDC 1802, Zasocitinib, AZD 4604, INCB-160058, Research programme: JAK2 inhibitors, Ritlecitinib, Momelotinib, DZD4205, ATI 2138, CPL 409116, Itacitinib, Ivarmacitinib, and others
Dive deep into rich insights for new JAK inhibitors, visit @ JAK Inhibitors Drugs
Table of Contents
| 1. | JAK Inhibitors Pipeline Report Introduction |
| 2. | JAK Inhibitors Pipeline Report Executive Summary |
| 3. | JAK Inhibitors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | JAK Inhibitors Clinical Trial Therapeutics |
| 6. | JAK Inhibitors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | JAK Inhibitors Pipeline: Late-Stage Products (Phase III) |
| 8. | JAK Inhibitors Pipeline: Mid-Stage Products (Phase II) |
| 9. | JAK Inhibitors Pipeline: Early-Stage Products (Phase I) |
| 10. | JAK Inhibitors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the JAK Inhibitors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the JAK Inhibitors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the JAK inhibitors pipeline therapeutics, reach out @ JAK Inhibitors Therapeutics
Related Reports
JAK Inhibitors Market Size, Target Population, Competitive Landscape & Market Forecast - 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key JAK inhibitors companies, including Incyte Corporation, Aclaris Therapeutics, Sareum, Takeda, AstraZeneca, Ajax Therapeutics, Pfizer, GSK, Dizal Pharmaceutical, Confluence Life Sciences, Celon Pharma, Arcutis Biotherapeutics, Reistone Biopharma, among others.
Janus Kinase Inhibitors Competitive Landscape
Janus Kinase Inhibitors Competitive Landscape – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key JAK inhibitors companies, including Pfizer, Sierra Oncology, Theravance Biopharma, Dizal Pharmaceutical, Aclaris Therapeutics, Celon Pharma, Incyte Corporation, AbbVie, Galapagos, Gilead Sciences, Reistone Biopharma, Jiangsu Hengrui Medicine Co., MaxiNovel Pharmaceuticals, among others.
Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atopic dermatitis companies including Bausch Health Companies Inc., GlaxoSmithKline PLC, Nestle SA, Pfizer Inc., Regeneron Pharmaceuticals Inc., Evelo Biosciences, Abbvie Inc., Allergan PLC, Cara Therapeutics, Bristol-Myers Squibb Company, Sanofi S.A., LEO Pharma, among others.
Atopic Dermatitis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key atopic dermatitis companies, including Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, Dermira, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, and others. Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, Vanda Pharmaceuticals, Kyowa Kirin, Sanofi, KeyMed Biosciences, Asana BioSciences, Bristol-Myers Squibb, RAPT Therapeutics, Allakos, Novartis, BioMimetix, Shanghai Hengrui Pharmaceutical Co, Connect Biopharma, Pfizer, Evommune, Inc., Fresh Tracks Therapeutics, Biosion, Chia Tai Tianqing Pharmaceutical, Reistone Biopharma Company Limited, JW Pharmaceutical, Oneness Biotech, Alphyn Biologics, selectION, UNION Therapeutics, Ichnos Scien, among others.
Vitiligo Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key vitiligo companies including Incyte Corporation, Amgen, Boston Pharmaceuticals, Arcutis Biotherapeutics, Pfizer, Dermavant Sciences, Clinuvel Pharmaceuticals, Celgene, TWi Biotechnology, AXIM Biotechnologies, Arrien Pharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

