5-HT2 Agonist Clinical Trial Pipeline Appears Robust With 20+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
Advancements in precision medicine drive the 5-HT2 agonist market by enabling selective targeting of specific receptor subtypes (e.g., 5-HT2A or 5-HT2C), reducing side effects and improving safety. Personalized approaches, like pharmacogenomics, identify patients most likely to benefit, enhancing treatment efficacy and adoption while accelerating drug development with cutting-edge technologies.
New York, USA, Jan. 22, 2025 (GLOBE NEWSWIRE) -- 5-HT2 Agonist Clinical Trial Pipeline Appears Robust With 20+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
Advancements in precision medicine drive the 5-HT2 agonist market by enabling selective targeting of specific receptor subtypes (e.g., 5-HT2A or 5-HT2C), reducing side effects and improving safety. Personalized approaches, like pharmacogenomics, identify patients most likely to benefit, enhancing treatment efficacy and adoption while accelerating drug development with cutting-edge technologies.
DelveInsight’s '5-HT2 Agonist Pipeline Insight 2025' report provides comprehensive global coverage of pipeline 5-HT2 agonists in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the 5-HT2 agonist pipeline domain.
Key Takeaways from the 5-HT2 Agonist Pipeline Report
- DelveInsight’s 5-HT2 agonist pipeline report depicts a robust space with 20+ active players working to develop 22+ pipeline 5-HT2 agonists.
- Key 5-HT2 agonist companies such as Cybin, Reunion Neuroscience, BetterLife Pharma, Gilgamesh Pharmaceuticals, Harmony Biosciences, Mindmed, ATAI LIFE SCIENCES N.V, Reviva Pharmaceuticals, Beckley Psytech, MindBio Therapeutics and others are evaluating new 5-HT2 agonist drugs to improve the treatment landscape.
- Promising pipeline 5-HT2 agonists such as CYB003, RE104, BETR-001, GM2505, EPX-100, MM120, EMP 01, Brilaroxazine, Psilocybin infusion, MB-22001, and others are under different phases of 5-HT2 agonist clinical trials.
- In December 2024, Mind Medicine announced that MM120 ODT, a pharmaceutically optimized form of lysergide D-tartrate (LSD), had been granted an Innovation Passport for the potential treatment of GAD under ILAP by the U.K. Medicines and Healthcare products Regulatory Agency (MHRA).
- In October 2024, Lundbeck A/S and Longboard Pharmaceuticals, Inc. announced an agreement for Lundbeck to acquire Longboard. Under the terms of the agreement, Lundbeck will commence a tender offer for all outstanding shares of Longboard common stock, whereby Longboard shareholders will be offered a payment of USD 60.00 per share in cash.
- In October 2024, Bright Minds Biosciences Inc. a pioneering company focused on developing highly selective 5-HT2 agonists for the treatment of drug-resistant epilepsy, depression, and other CNS disorders, announced positive data from the preclinical testing of BMB-201 completed with National Institute of Health pain screening (PSPP) program.
- In March 2024, Mind Medicine announced that FDA has granted breakthrough designation to its MM120 (lysergide d-tartrate) program for the treatment of generalized anxiety disorder (GAD). the company also announced that its Phase 2b study of MM120 in GAD met its key secondary endpoint, and 12-week topline data demonstrated clinically and statistically significant durability of activity observed through Week 12.
Request a sample and discover the recent advances in 5-HT2 agonist drugs @ 5-HT2 Agonist Pipeline Report
The 5-HT2 agonist pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage 5-HT2 agonist drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the 5-HT2 agonist clinical trial landscape.
5-HT2 Agonist Overview
5-HT2 (5-hydroxytryptamine 2) agonists are a class of drugs that selectively activate 5-HT2 receptors, a subgroup of serotonin receptors located in the central nervous system. Serotonin also referred to as 5-hydroxytryptamine (5-HT), is a neurotransmitter that plays a critical role in regulating mood, cognition, and sensory processing. The 5-HT2 receptor family includes three main subtypes: 5-HT2A, 5-HT2B, and 5-HT2C.
By binding to and stimulating these receptors, 5-HT2 agonists initiate a series of intracellular signaling cascades. These receptors, which are G protein-coupled receptors (GPCRs), influence key signaling pathways such as phospholipase C (PLC), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK). As integral membrane proteins with seven transmembrane domains, 5-HT2 receptors mediate serotonin's effects by facilitating ligand binding, G protein coupling, and activation of downstream signaling, resulting in a variety of physiological and pharmacological outcomes. Understanding these mechanisms is crucial for developing therapeutic drugs targeting 5-HT2 receptors and for exploring their roles in health and disease.
Therapeutic targeting of 5-HT2 receptors has shown promise in several medical fields due to their significant role in regulating physiological processes. In psychiatry, 5-HT2A agonists have demonstrated potential as treatments for depression and anxiety, with compounds like psilocybin (from psychedelic mushrooms) and lysergic acid diethylamide (LSD) showing efficacy in controlled clinical settings. Similarly, 5-HT2C agonists are being investigated for their ability to manage obesity and metabolic disorders by modulating appetite and energy balance. In neurology, 5-HT2B and 5-HT2C agonists are being studied for their vasoconstrictive effects in migraine management, while 5-HT2B agonists are also under exploration for their cardioprotective benefits in conditions such as heart failure.
Despite their potential, the clinical application of 5-HT2 agonists requires careful consideration of their psychotropic effects and possible side effects. This highlights the need for rigorous research and controlled usage to optimize their therapeutic advantages.
Find out more about 5-HT2 agonist drugs @ 5-HT2 Agonist Analysis
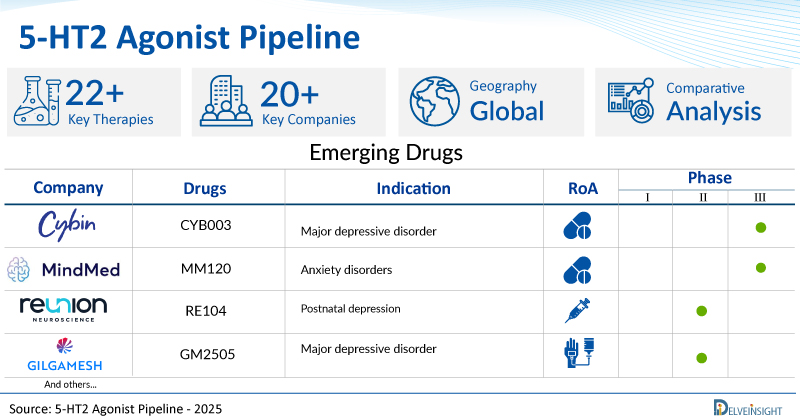
A snapshot of the Pipeline 5-HT2 Agonist Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| CYB003 | Cybin | III | Major depressive disorder | Oral |
| MM120 | MindMed/The University Hospital of Basel | III | Anxiety disorders | Oral |
| RE104 | Reunion Neuroscience | II | Postnatal depression | Subcutaneous |
| GM2505 | Gilgamesh Pharmaceuticals | II | Major depressive disorder | Intravenous |
| EPX-100 | Epygenix Therapeutics/University of California at San Fransisco | II | Dravet syndrome; Lennox-Gastaut syndrome | Oral |
| BETR-001 | BetterLife Pharma | Preclinical | Cluster headache; Major depressive disorder; Neuropathic pain; Post-traumatic stress disorders | NA |
Learn more about the emerging 5-HT2 agonist @ 5-HT2 Agonist Clinical Trials
5-HT2 Agonist Therapeutics Assessment
The 5-HT2 agonist pipeline report proffers an integral view of the emerging 5-HT2 agonist segmented by stage, product type, molecule type, and route of administration.
Scope of the 5-HT2 Agonist Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Key 5-HT2 Agonist Companies: Cybin, Reunion Neuroscience, BetterLife Pharma, Gilgamesh Pharmaceuticals, Harmony Biosciences, Mindmed, ATAI LIFE SCIENCES N.V, Reviva Pharmaceuticals, Beckley Psytech, MindBio Therapeutics and others,
- Key 5-HT2 Agonist Pipeline Therapies: CYB003, RE104, BETR-001, GM2505, EPX-100, MM120, EMP 01, Brilaroxazine, Psilocybin infusion, MB-22001 and others.
Dive deep into rich insights for new 5-HT2 agonists, visit @ 5-HT2 Agonist Drugs
Table of Contents
| 1. | 5-HT2 Agonist Pipeline Report Introduction |
| 2. | 5-HT2 Agonist Pipeline Report Executive Summary |
| 3. | 5-HT2 Agonist Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | 5-HT2 Agonist Clinical Trial Therapeutics |
| 6. | 5-HT2 Agonist Pipeline: Late-Stage Products (Pre-registration) |
| 7. | 5-HT2 Agonist Pipeline: Late-Stage Products (Phase III) |
| 8. | 5-HT2 Agonist Pipeline: Mid-Stage Products (Phase II) |
| 9. | 5-HT2 Agonist Pipeline: Early-Stage Products (Phase I) |
| 10. | 5-HT2 Agonist Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the 5-HT2 Agonist Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the 5-HT2 Agonist Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the 5-HT2 agonist pipeline therapeutics, reach out @ 5-HT2 Agonist Therapeutics
Related Reports
Major Depressive Disorder Epidemiology Forecast
Major Depressive Disorder Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted major depressive disorder epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Major Depressive Disorder Market
Major Depressive Disorder Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key MDD companies, including Otsuka Pharmaceutical Development & Commercialization, Inc., Sumitomo Pharma Co., Ltd., COMPASS Pathways, Chase Therapeutics Corporation, Cybin IRL Limited, Neumora Therapeutics, Inc., BioLite, Inc., Sage Therapeutics, Xenon Pharmaceuticals Inc., Neurocrine Biosciences, Ancora Bio, Inc. d/b/a EmbarkNeuro, Inc., Relmada Therapeutics, Inc., Intra-Cellular Therapies, Inc., Alto Neuroscience, Tonix Pharmaceuticals, Inc., Janssen Research & Development, LLC, Boehringer Ingelheim, among others.
Major Depressive Disorder Pipeline
Major Depressive Disorder Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key major depressive disorder companies, including GH Research, Praxis Precision Medicines, AbbVie, Gedeon Richter, Intra-Cellular Therapies, Bristol-Myers Squibb, Relmada Therapeutics, SAGE Therapeutics, Janssen Research & Development, Minerva Neurosciences, Takeda, Neurocrine Biosciences, Pherin Pharmaceuticals, among others.
Postpartum Depression Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key postpartum depression companies, including Sage Therapeutics, Epharmix, Inc., among others.
Treatment-Resistant Depression Pipeline
Treatment-Resistant Depression Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key treatment-resistant depression companies, including Axsome Therapeutics, COMPASS Pathways, Merck Sharp & Dohme Corp., Navitor Pharmaceuticals, Inc., Taisho Pharmaceutical Co., Ltd., GH Research Limited, Novartis Pharmaceuticals, Reckitt Benckiser LLC, Relmada Therapeutics, Inc., SAGE Therapeutics, Navitor Pharmaceuticals, Sumitomo Dainippon Pharma, Alkermes, AbbVie, Janssen Pharmaceuticals, Celon Pharma, ACADIA Pharmaceuticals, Pherin Pharmaceuticals, ATAI Life Sciences, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +91-9650213330 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

