Comprehensive Market Analysis of Latest Published 9 Rare Genetic Disorders Reports | DelveInsight
Rare genetic disorders affect approximately 1 in 10 people worldwide, meaning over 350 million individuals are living with these conditions. Despite their rarity, there are over 7,000 identified rare diseases, and 80% of them are genetic in origin, often manifesting early in life.
New York, USA, Dec. 19, 2024 (GLOBE NEWSWIRE) -- Comprehensive Market Analysis of Latest Published 9 Rare Genetic Disorders Reports | DelveInsight
Rare genetic disorders affect approximately 1 in 10 people worldwide, meaning over 350 million individuals are living with these conditions. Despite their rarity, there are over 7,000 identified rare diseases, and 80% of them are genetic in origin, often manifesting early in life.
Rare genetic disorders, often caused by mutations in a single gene or a combination of genetic factors, affect a small percentage of the population but collectively impact millions worldwide. Despite advances in genetic research and precision medicine, there remains a significant treatment gap. Many rare diseases are under-researched due to their low prevalence, which limits pharmaceutical investment and drug development.
As a result, patients face limited treatment options, delayed diagnoses, and inadequate access to specialized care. Bridging this gap requires increased funding, collaborative global research, and innovative approaches like gene therapies, RNA-based treatments, and patient-centered clinical trials to provide hope and equitable healthcare solutions for affected individuals.
DelveInsight has expertise in the rare disease market with an experienced team handling the rare disease domain proficiently. DelveInsight has recently released a series of epidemiology-based market reports on rare genetic disorders including Usher Syndrome, Alport Syndrome, Menkes Disease, Molybdenum Cofactor Deficiency Type-A, Prader-Willi Syndrome, Osteogenesis Imperfecta, Myotonic Dystrophy, Alpha-1 Antitrypsin Deficiency, and Neurofibromatosis type 1-associated Plexiform Neurofibromas. These reports include a comprehensive understanding of current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034 segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Additionally, the reports feature an examination of prominent companies working with their lead candidates in different stages of clinical development. Let’s deep dive into the assessment of these rare genetic disorders markets individually.
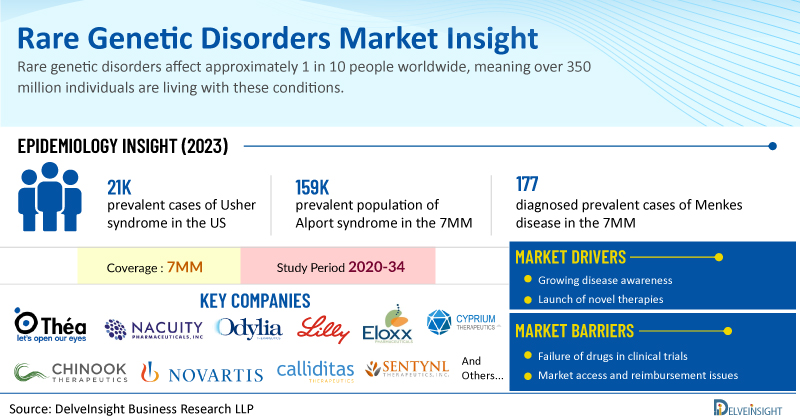
Usher syndrome is the most common cause of deaf-blindness, accounting for half of the cases in individuals under 65. This genetic disorder presents as a combination of hearing impairment, retinopathy, and vestibular dysfunction, with each symptom varying in form and onset. Among the 7MM, the US accounted for the highest prevalent cases of Usher syndrome in 2023, with around 21K cases; these cases are expected to increase during the forecast period.
At present, LUXTURNA (voretigene neparvovec) is the only approved treatment for Usher Syndrome, specifically for a small subset of patients with the RPE65 mutation. However, no standard therapy exists for patients without this mutation, leaving most to depend on supportive care such as vitamin supplements, sun protection, and visual aids.
According to DelveInsight’s analysis, the market for Usher syndrome in the 7MM will grow from USD 76.48 million in 2023 at a CAGR of 10.55% by 2034. This growth is mainly due to the anticipated launch of emerging therapies in the coming years.
Usher Syndrome Pipeline Therapies and Key Companies
- ULTEVURSEN: LABORATOIRES THÉA
- NPI-001: NACUITY PHARMACEUTICALS
- RESEARCH PROGRAM: DUAL ADENO-ASSOCIATED VIRUS BASED USHER SYNDROME TYPE 1B GENE THERAPY: AAVANTGARDE BIO
- AAV- USH1C: ODYLIA THERAPEUTICS
- AK-CLRN1: ELI LILLY AND COMPANY
- ATSN-301: ATSENA THERAPEUTICS
- RESEARCH PROGRAM: GENE THERAPY: CLEARSIDE BIOMEDICAL
Learn more about the FDA-approved drugs for Usher syndrome @ Drugs for Usher Syndrome Treatment
Alport syndrome is a rare genetic condition that affects the inner ear and eyes, resulting from an inherited defect in type IV collagen. This structural protein is essential for the proper functioning of various body tissues. The disorder can manifest in a range of different forms.
Alport syndrome is an inherited disease, with X-linked being the most common type of it, and accounts for approximately 85% of the total cases. DelveInsight’s analysis reveals that the overall prevalent population of Alport syndrome in the 7MM was reported as 159K in 2023.
ACE inhibitors and ARBs currently serve as the cornerstone of treatment for CKD in Alport syndrome. However, recent years have seen a significant surge in efforts to develop new therapies. Several well-established, patient-founded organizations dedicated to Alport syndrome now play a pivotal role in supporting and advancing clinical trials, to discover innovative treatment options.
While existing treatments can help slow kidney disease progression in Alport syndrome, there remains no cure, nor has any therapy been proven to completely halt kidney decline. As per DelveInsight analysis, the market in the 7MM is expected to surge from USD 20 million in 2023 at a massive CAGR of 69% by 2034.
Over the next decade, advancements in early diagnosis and treatment are expected to delay the onset of kidney failure in affected individuals. Furthermore, emerging therapies designed to complement ACE inhibitors are anticipated to provide additional benefits. Although the development of safe and effective curative treatments is a possibility, overcoming significant challenges will be essential to turn these aspirations into reality.
Alport Syndrome Pipeline Therapies and Key Companies
- ELX-02: Eloxx Pharmaceuticals
- Atrasentan: Chinook Therapeutics/Novartis
- Finerenone: Bayer
- Setanaxib: Calliditas Therapeutics
- BAY3401016: Evotec/Bayer
For a comprehensive view of the Alport syndrome market, check out the Alport Syndrome Market Assessment
Menkes disease is a fatal neurodegenerative disorder that occurs in infants and follows an X-linked inheritance pattern. It is an X-linked recessive condition caused by mutations in the ATP7A gene, which regulates copper transport in the body. The quality of life (QoL) of individuals with Menkes disease declines quickly due to the progressive worsening of symptoms and outcomes. As per our assessment, in 2023, nearly 177 diagnosed prevalent cases of Menkes disease were estimated in the 7MM. These cases are anticipated to increase by 2034. The US accounted for the highest cases.
The progression of Menkes disease is unpredictable and rapid, often requiring medical intervention. Unfortunately, the prognosis for affected patients is poor, with survival typically not exceeding 3.5 years unless copper histidine or other copper replacement therapies are available.
Currently, management of Menkes disease relies on standard care, including off-label copper supplements (such as copper histidine or copper chloride), antiepileptic drugs, L-threo-dihydroxyphenylserine, and other treatments. Advancements in therapeutic approaches have paved the way for emerging therapies. New treatments, such as CUTX-101, are currently under development for Menkes disease.
At present, CUTX-101 appears to be the most promising candidate in the Menkes disease clinical pipeline. The market is expected to respond positively to CUTX-101, reinforcing its potential as a leading therapy for the condition, as no other therapy has yet achieved approval. We anticipate that the expected approval of emerging therapies and greater awareness of early disease screening will drive significant market growth in the 7MM from USD 7.9 million in 2023.
Menkes Disease Pipeline Therapies and Key Companies
- CUTX-101: Cyprium Therapeutics/Sentynl Therapeutics
To gain a deeper understanding of the Menkes disease market, be sure to explore the Menkes Disease Market Outlook
Molybdenum Cofactor Deficiency Type-A Market
Molybdenum cofactor deficiency (MoCoD) is a rare metabolic condition marked by severe, worsening neurological damage, disrupted autonomic function, heightened startle responses, abnormal facial features, changes in muscle tone, progressive cerebral palsy, microcephaly, seizures, and early death. It results from the loss of function of sulfite oxidase, one of the four molybdenum-dependent enzymes in humans.
DelveInsight’s analysts estimate that approximately 270 prevalent cases of MOCOD-A were found in 2023 in the 7MM. The United States exhibited the highest diagnosed prevalent cases of MOCOD-A, as compared to other 7MM countries.
The treatment of MoCoD-A is difficult because of its rarity and the need for specialized care. The main treatment goal is symptom management and preventing complications. MoCoD-A with severe neonatal symptoms has limited therapeutic options, and the prognosis is typically poor.
Many infants require intubation for ventilation support due to seizures or impaired mental status. Generally, the approach involves informing parents and caregivers of the very poor prognosis and offering the option to refrain from aggressive resuscitation efforts or withdraw life-support measures.
Attributed to the rising diagnosed prevalent cases and increasing awareness, the MoCoD-A market was valued at nearly USD 12 million in 2023 and is expected to experience consistent growth during the forecast period (2024–2034).
Discover which therapies are expected to grab the MoCoD-A market share @ MoCoD-A Market Report
Prader-Willi syndrome is a rare genetic condition that impacts individuals of both sexes from birth and throughout their lives. It is marked by reduced muscle tone, delays in motor development, mild to moderate cognitive impairments, incomplete sexual maturation, and emotional and social immaturity, often resulting in challenging behaviors.
According to DelveInsight’s analysis, there were 24,990 diagnosed prevalent cases of Prader-Willi syndrome in the 7MM in 2023. While several studies suggest a higher average diagnosis rate, ranging from 65-80%, real-world evidence from registries indicates a somewhat larger diagnosis gap.
Despite the current disparity, the number of diagnosed cases is expected to increase during the forecast period (2024−2034) due to advancements in diagnostic technology, greater awareness of the condition, and the establishment of more PWS registries.
Treatment options for Prader-Willi syndrome are currently limited and primarily focus on lifestyle modifications to reduce the risk of obesity-related deaths. Nearly half of the deaths in patients with Prader-Willi syndrome under 18 are linked to food-seeking behaviors, such as choking and accidents. The FDA has approved three growth hormone treatments for the condition: GENOTROPIN by Pfizer, NORDITROPIN by Novo Nordisk, and OMNITROPE by Sandoz. This approval enables healthcare providers to prescribe these therapies as alternatives.
According to DelveInsight’s analysis, the market size of Prader–Willi syndrome in the 7MM reached USD 600 million in 2023 and is expected to increase by 2034 at a CAGR of ~6%, majorly due to the expected drastic YoY growth in diagnosis rate, and the high cost of the expected launch of several key therapies.
Prader-Willi Syndrome Pipeline Therapies and Key Companies
- WAKIX (pitolisant): Harmony Biosciences
- Diazoxide Choline Controlled-Release (DCCR): Soleno Therapeutics
- Carbetocin (ACP-101, LV-101): Acadia Pharmaceuticals
- ARD-101: Aardvark Therapeutics
- RGH-706: Gedeon Richter
- PBF-999: Palobiofarma
- Tesomet: Saniona
To access a complete analysis of the Prader-Willi syndrome market, visit Prader-Willi Syndrome Market Assessment
Osteogenesis Imperfecta Market
Osteogenesis imperfecta refers to a group of genetic disorders primarily impacting bone development. Individuals with osteogenesis imperfecta have fragile bones that fracture easily, even with minimal or no trauma. There are at least 19 identified types of osteogenesis imperfecta, ranging from type I to type XIX. While each type is characterized by specific signs and symptoms, many of their features overlap. In 2023, there were about 72K prevalent cases of osteogenesis imperfecta across the 7MM. In 2023, the US accounted for ~55% of all prevalent cases of osteogenesis imperfecta.
Osteogenesis imperfecta treatment aims to prevent fractures and alleviate symptoms, as there is no cure for the condition. Bisphosphonates are often prescribed to improve bone strength and reduce the risk of fractures. Additional treatments may include physical therapy, orthopedic surgery, and assistive devices to improve mobility and independence. In more severe cases, surgeries such as rodding may be used to stabilize long bones and prevent deformities.
In 2023, the osteogenesis imperfecta market across the 7MM was approximately USD 30 million. The market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of therapies, and raised awareness.
Osteogenesis Imperfecta Pipeline Therapies and Key Companies
- EVENITY (romosozumab): Amgen/UCB
- Setrusumab (UX143): Ultragenyx Pharmaceuticals/Mereo BioPharma
Dive deeper for rich insights into the Osteogenesis Imperfecta Clinical Trials
Myotonic dystrophy is a type of muscular dystrophy, a category of disorders marked by the weakening and deterioration of voluntary muscles throughout the body. Each form of muscular dystrophy presents unique abnormalities, including differences in muscle fiber size, muscle fiber death, the formation of scar tissue, and inflammation, all of which can be seen in muscle biopsies from affected individuals. As per DelveInsight’s estimations, the total diagnosed prevalent cases of myotonic dystrophy in the 7MM was approximately 105K cases in 2023 and are projected to decrease during the forecast period.
Currently, there is no approved therapy to cure or slow the progression of myotonic dystrophy, though symptomatic treatments are available. These treatments aim to alleviate symptoms and improve quality of life (QoL). Among the symptoms of myotonic dystrophy, myotonia is the most common, and it is typically treated with antimyotonic agents such as mexiletine, lamotrigine, carbamazepine, oxcarbazepine, flecainide, propafenone, phenytoin, and ranolazine, all of which are prescribed off-label.
For chronic muscle pain, the pharmacological approach follows the WHO’s four-step ladder, starting with NSAIDs, and, if necessary, adding adjuvant therapies like anticonvulsants (pregabalin, gabapentin), antidepressants (duloxetine, amitriptyline, nortriptyline), muscle relaxants (baclofen, tizanidine), or topical agents (lidocaine or capsaicin patches).
The market for therapeutics targeting myotonic dystrophy is projected to experience robust growth, at a significant CAGR of 18.4% from 2020 to 2034. This dynamic expansion reflects increasing demand for advanced treatment options and underscores the growing investment in addressing this serious condition.
Furthermore, the myotonic dystrophy market is influenced by key factors, such as the rising incidence of genetic disorders and increased awareness of rare diseases. Progress in genomic research and growing investments in rare disease treatments are fueling market expansion. However, challenges like limited research funding, high costs of new therapies, and stringent regulatory requirements continue to pose significant barriers.
Additionally, the substantial unmet need for effective Myotonic Dystrophy treatments highlights a major gap in current therapies, offering a valuable opportunity for companies to innovate and meet the diverse needs of patients.
Myotonic Dystrophy Pipeline Therapies and Key Companies
- AMO-02 (tideglusib): AMO Pharma Limited
- Mexiletine: Lupin Ltd.
- Pitolisant: Harmony Biosciences, LLC
- Delpacibart etedesiran (AOC-1001): Avidity Biosciences, Inc.
For a deeper understanding of the myotonic dystrophy market landscape, explore the Myotonic Dystrophy Market Outlook
Alpha-1 Antitrypsin Deficiency Market
Alpha-1 antitrypsin deficiency (AATD) is an inherited condition marked by insufficient levels of a protein called alpha-1 antitrypsin (AAT) in the blood. This deficiency can increase the risk of various health issues, most commonly presenting as COPD, including bronchiectasis, and liver diseases such as cirrhosis and hepatoma. In rarer cases, it can also lead to a skin disorder known as panniculitis.
The total prevalent cases of AATD in the 7MM were ~224K in 2023. The prevalence of AATD in Japan is significantly lower than in Europe and the United States. As per estimates, in 2020, in the US, comorbidity associated with lung diseases was most common, occurring in around 77% of the total AATD cases, followed by other diseases and liver diseases.
The current treatment options do not offer a cure. Augmentation therapy, also known as replacement therapy, is approved for the treatment of alpha-1-related lung disease. In the US, four augmentation therapy products are available: Grifols’ PROLASTIN-C, Takeda’s ARALAST, CSL Behring’s ZEMAIRA, and Kamada/Takeda’s GLASSIA.
In certain European countries, AATD patients have access to RESPREEZA (ZEMAIRA in the US), PROLASTIN, PROLASTINA, PROLASPLAN, PLITALFA, and ALFALASTIN. In Japan, PROLASTIN-C is sold under the name LYNSPAD. PROLASTIN, produced by Grifols, is the market leader in AATD augmentation therapies, according to 2023 sales data.
The pipeline for AATD consists an innovative therapies including an immunomodulator (serine peptidase inhibitor), neutrophil elastase enzyme inhibitor, RNA editing therapy, gene therapies, and others. As per DelveInsight analysis, among the 7MM, the US accounted for the largest market size of AATD. i.e., USD ~700 million in 2023.
Ongoing research and an increase in disease understanding have led to the identification of therapies with effective and convenient routes of administration, including subcutaneous, inhalation, and oral, with the potential to improve patient’s quality of life, setting the stage for market expansion and reshaping patient expectations and treatment experiences.
AATD Pipeline Therapies and Key Companies
- Inhaled Alpha 1-Antitrypsin (AAT): Kamada Pharmaceuticals
- Fazirsiran (ARO-AAT/TAK-999): Arrowhead Pharmaceuticals and Takeda
- Alvelestat (MPH-966): Mereo BioPharma/AstraZeneca
- SAR447537/INBRX-101: Sanofi/Inhibrx Biosciences
- WVE-006: Wave Life Sciences
- BEAM-302: Beam Therapeutics
- Alpha-1 AT 15% (SC): Grifols
- KB408: Krystal Biotech
To access a complete analysis of the AATD market, visit Alpha-1 Antitrypsin Deficiency Market Assessment
Neurofibromatosis type 1-associated Plexiform Neurofibromas Market
NF1 is the most prevalent tumor predisposition syndrome, resulting from mutations in the NF1 gene. These mutations lead to a deficiency of neurofibromin, a regulator of RAS activity, which in turn causes the formation of plexiform neurofibromas—peripheral nerve sheath tumors that significantly affect the health and quality of life of those affected.
In 2023, the total diagnosed prevalent cases of NF1 were around 97K in the US, and these cases are anticipated to increase by 2034. The rise in NF1 cases can be attributed to improved awareness, advancements in diagnostic techniques, and increased genetic testing accessibility. Among the 7MM, the US accounted for the highest number of cases in 2023, with around 39K diagnosed prevalent cases of NF1-PN. These cases are expected to increase during the forecast period.
The treatment options for NF1-PN have traditionally been limited to surgical procedures, primarily complete resection or debulking. However, the tumors’ size and location often make these methods challenging, with many considered inoperable due to their proximity to critical structures or their invasive nature. Around 50% of patients with NF1-PN have tumors that cannot be safely removed without significant risk of complications, requiring a focus on managing symptoms rather than offering curative surgery.
Surgery remains the preferred option for NF1-PN when possible. While complete resection is ideal, it is rarely achievable due to the tumors' complex relationship with nerves and blood vessels. Debulking may reduce symptoms but does not ensure long-term relief, as the tumors often regrow. The decision to pursue surgery depends on a comprehensive assessment of the tumor's characteristics and the patient’s overall health. The approval of KOSELUGO, a selective MEK1/2 inhibitor, in 2020 represented a major shift in the treatment approach for pediatric patients with symptomatic, inoperable NF1-PN. KOSELUGO is currently the only FDA-approved treatment specifically for this condition.
The total market size of NF1-PN in the 7MM was around USD 380 million in 2023. This is estimated to increase by 2034. Among the forecasted emerging therapies, mirdametinib is expected to capture the highest market in the 7MM by 2034. The landscape for treating NF1-PN is evolving rapidly as research continues to uncover the genetic underpinnings of these tumors.
NF1-PN Pipeline Therapies and Key Companies
- MIRDAMETINIB (PD-0325901): SPRINGWORKS THERAPEUTICS
- FCN-159: FOSUN PHARMACEUTICAL
- HLX-1502: HEALX
Discover more about NF1-PN drugs in development @ NF1-PN Clinical Trials
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Rare Disease Consulting Services
Delveinsight’s comprehensive rare disease consulting services encompass rare disease consulting, epidemiology-based market assessment, and primary research projects aimed at obtaining elusive data through their esteemed KOL panel.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

